A. Overview of Acute Treatment
1. Indications
The diagnosis of KD is based on the diagnostic guidelines for KD (6th revised edition).14) KD is diagnosed when five or more of the six principal clinical features are present during the course of the disease. In complete KD with fever, standard treatment including IVIG and aspirin (ASA) is recommended. According to the guideline, patients with four major symptoms who exhibit CAA on echocardiography can be diagnosed with KD, if other diseases are ruled out. If there are four major symptoms without CAA or less than three major symptoms, the diagnosis is incomplete KD, and differential diagnoses are sought. Incomplete KD has been reported to have an equivalent risk of CAA complications as complete KD (higher in the Nationwide Survey).15) Standard treatment is recommended in cases of incomplete KD after ruling out other diseases.
2. Targets for Treatment Based on Pathology
Based on the definition of systematic vasculitis syndrome, KD is classified as medium-sized vasculitis.16) Inflammation is also found in the aorta and small arteries, but it occurs mainly in the coronary arteries of medium-sized muscular arteries. According to the histopathological study of coronary arteritis,17) on day 6 to 8 of illness, the tunica media is dissected due to edema, and the inflammatory cells, mainly monocytes/macrophages, are confined to the inner and outer membranes. On day 8 to 10 of illness, inflammatory cells infiltrate the tunica media from the intima and adventitia, causing panarteritis involving the entire arterial wall. Cytokines, proteolytic enzymes, and reactive oxygen species, all of which are produced by inflammatory cells, cause rupture of the internal elasticity plates and injury to the smooth muscle, leading to vulnerability of blood vessels. On day 10 to 12 of illness, blood pressure is increased to the vulnerable vessels, and they expand centrifugally to form CAA, like a balloon inflating.
Since the goal of acute KD treatment is to terminate inflammation and reduce the development of CAA, it is recommended that IVIG be administered no later than the 7th day of illness before the onset of panarteritis. Even if patients are unresponsive to IVIG and have persistent or recurrent fever, treatment should aim for success at up to the 9th day of illness, before coronary artery expansion generally begins.2), 3) Additional treatment after the onset of CAA may fail to inhibit dilation, and hence, early judgement of unresponsiveness to IVIG is encouraged.
3. Policy of Acute Treatment
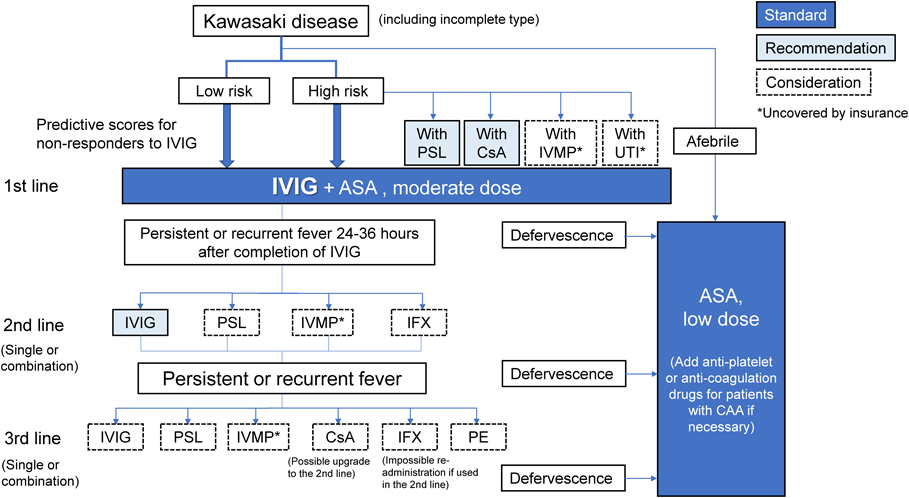
(1) The Therapeutic Algorithm (Fig. 1)
Based on the results of new studies, the algorithm for acute treatment in the previous guidelines3) has been revised. For the revision, by referring to the classifications and evidence level of each treatment, as well as regulatory approval and insurance coverage, we placed treatments into three categories: standard treatment, recommended treatment, and treatment to be considered in each time phase (denoted as “line” hereafter). Other than the initial treatment with IVIG and ASA, the superiority of indication, usage, combination, and order has not been well established. It is therefore desirable to select treatments in each institution according to the classifications and the evidence levels in the text for the actual treatment. Although empirical therapies at each institution are not restricted in the guidelines, when performing them, informed consent must be obtained from patients and their families, and approval by the research review committee or the ethics committee is necessary.
(2) Initial Treatment
①Standard Treatment
The first line of initial treatment consists of standard treatment with IVIG, drip infusion of 2 g/kg, and a medium dose of ASA (30–50 mg/kg/day), if fever is present (Class I, Level of evidence A; denoted as “level” hereafter). In the absence of fever, ASA (5 mg/kg/day) may be administered orally, but careful course observation is required, and it is advisable to consider the administration of IVIG based on the diagnosis of so-called indolent or smoldering type,18) if low-grade fever persists, there is worsening of the inflammatory response on blood tests, or signs of CAA appear on echocardiography.
If patients become afebrile after the initial treatment and do not show recurrent fever, the dose of ASA is reduced to 3 to 5 mg/kg/day. At 24 to 36 h after completion of IVIG administration, if fever (axillary temperature of 37.5°C or more, or core body temperature of 38.0°C or more) is present, additional second-line treatments are recommended based on the judged unresponsiveness.19) Non-responders to IVIG were judged 24 h after completion of IVIG treatment in the previous guidelines; however, in light of the current situation where the administration time differs due to the increase in products, the timing for determining the response is 24 to 36 h after completion of treatment19) (basically 48 h after the start of treatment) in the present guidelines. It is desirable to determine the necessity of additional treatments by considering symptoms other than fever and blood tests. Even once fever has been relieved, additional second-line or more treatments are advisable for recurrent cases with exacerbation or re-emergence of the principal clinical features similar to unresponsive cases, if other febrile diseases are unlikely.
②Initial Combination Therapy
In Japan, risk scores to predict responsiveness to standard treatment have been proposed before the start of initial IVIG, based on a combination of age, illness day of diagnosis, and blood test results (Table 1).20–22) Combined treatments for predicted non-responders to IVIG are the usual dose of PSL (starting at 2 mg/kg/day and tapering off)4) or CsA (5 mg/kg/day for 5 days)10) for patients with a high-risk Kobayashi score20) and IVMP (30 mg/kg/dose, once)23, 24) for those with a high-risk Egami score21) or Sano score.22) The combination of these two drugs with IVIG reduced the incidences of unresponsive cases and CAA. In spite of a retrospective study, the combination of UTI (5000 units/kg/time, 3–6 times/day) was reported to be similarly effective.25)
Table 1 Representative scoring systems for predicting non-responders to IVIG| 1. Kobayashi (Gunma) score20): ≥ 5 points; sensitivity 76%, specificity 80% |
|---|
| Cut-off point | Points |
|---|
| Sodium ≤133 mEq/L | 2 |
| Timing of initial IVIG ≤4 days of illness | 2 |
| AST ≥100 IU/L | 2 |
| Neutrophil ratio ≥80% | 2 |
| CRP ≥10 mg/dL | 1 |
| Platelet counts ≤300,000/mm3 | 1 |
| Age ≤12 months | 1 |
| 2. Egami (Kurume) score21): ≥ 3 points; sensitivity 78%, specificity 76% |
|---|
| Cut-off point | Points |
|---|
| ALT ≥80 IU/L | 2 |
| Timing of initial IVIG ≤4 days of illness | 2 |
| CRP ≥8 mg/dL | 2 |
| Platelet counts ≤300,000/mm3 | 2 |
| Age ≤6 months | 1 |
| 3. Sano (Osaka) score22): ≥ 2 points; sensitivity 77%, specificity 86% |
|---|
| Cut-off point | Points |
|---|
| AST ≥200 IU/L | 1 |
| Total bilirubin≥0.9 mg/dL | 1 |
| CRP ≥7 mg/dL | 1 |
| AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, CRP: C-reactive protein, IVIG: intravenous immunoglobulin |
In the present guidelines, therefore, for predicted non-responders to IVIG, combined use of insurance-covered PSL (class I, level A) or CsA (class IIa, level B) is recommended. Although not covered by insurance, combined use of IVMP (Class IIa, Level B) or UTI (Class IIb, Level C) may be considered. Poor reproducibility of the prediction scores developed in Japan is a problem for foreign countries. In the United States, a method of predicting CAA 6 weeks after onset was proposed using coronary artery enlargement (Z score of internal diameters, 2.0–2.5 or greater) and other factors before initial treatment,26) and combined use of PSL or IFX with standard treatment was reported to be effective for such cases.27)
③Additional Treatment
Re-administration of IVIG is recommended as an additional second-line treatment (Class I, Level C). PSL (Class IIa, Level C in combination with IVIG), IVMP (Class IIa, Level B), and IFX (5 mg/kg/dose; Class IIa, Level B) alone or with IVIG may be considered. If PSL, CsA, or UTI is used in combination with initial IVIG, other additional treatments are performed during continuation of these drugs in many cases. Although there is insufficient evidence, CsA can be used as an additional second-line treatment (Class IIb, Level C) for patients who did not receive CsA for the initial treatment. If patients become afebrile without recurrent fever after completion of additional second-line treatment, ASA is reduced to a low dose (3–5 mg/kg/day).
If fever persists or recurs, for additional third-line treatments, IVIG, PSL, IVMP, CsA, IFX, or plasma exchange (PE; Class IIa, Level C) may be considered. IFX should not be re-administered in the third and subsequent treatment lines for patients who received it as a second-line treatment (Class IIb, Level C). IVIG is an option for additional treatment in any treatment lines, but another drug is worth considering in third-line treatment if it has not yet been used in the first- or second-line treatment. It is advisable to select the treatments presented in the present guidelines, depending on the patient’s condition, if they do not respond to third-line treatment.
④Complementary Treatment
Appropriate complementary treatments are recommended for cardiovascular complications other than CAA, such as myocarditis, pericarditis, valvular disease, arrhythmia, and shock, depending on the condition. In case of heart failure, edema,28) and hyponatremia due to syndrome of inappropriate secretion of antidiuretic hormone secretion (SIADH),29) the fluid infusion volume must be carefully monitored to avoid overloading the body with fluids. In contrast, adequate fluids are necessary if there are signs of dehydration. Symptomatic treatment is also important for systemic organ complications, including paralytic ileus, cholecystitis, pancreatitis, encephalitis/encephalopathy, and hemophagocytic syndrome.
So-called Kawasaki disease shock syndrome (KDSS) is a disease associated with low blood pressure and requiring intensive treatments.30, 31) KDSS is prevalent in older children and females, involves high inflammatory response, thrombocytopenia, and hyponatremia, and is often accompanied by CAA with unresponsive to IVIG. Accordingly, initial reinforcement therapies and strict systemic management with the expectation of additional treatments are necessary. In addition, general treatments for shock, such as infusion of isotonic crystalloid solution, catecholamines, and ventilators, are also performed. Because reported cases are mainly from Europe and the United States, but rarely from Japan, KDSS may be related to racial differences, and its essence and appropriate treatments are a subject for further studies.
4. Actual State of Acute Treatments in the Nationwide Survey
According to the 25th Nationwide Survey of KD,1) 94.6% of patients were treated with IVIG as initial therapy. The initial combination of steroids was used in 13.2% of patients, of which, PSL and IVMP were used in 84.6% and 17.1%, respectively. As additional treatments for non-responders to initial IVIG, 21.6% of patients were treated with IVIG, 6.3% with steroids, 2.6% with IFX, 1.5% with CsA (referred to as immunosuppressive agents), and 0.5% with PE. Cardiac abnormalities in the acute phase less than 1 month after onset were 7.6% CAA with giant aneurysms (0.1%) and enlargement (6.5%), and 8.9% including coronary artery stenosis, myocardial infarction, and valvular lesions. The cardiac sequelae 1 month after onset were CAA, including giant aneurysms (0.1%) and enlargement (1.5%), which accounted for 2.2% of all cases. When coronary artery stenosis, myocardial infarction, and valvular lesions were included, the rate was 2.6%. There were six deaths (0.002%), and the mortality rate was decreased compared to previous data, but it should be noted that a small number of patients still died.
B. Health Economics
As the treatment of KD often requires expensive treatments, such as IVIG, reports on acute inpatient medical care costs have been published in several countries.
In a study of 7,431 American children under 5 years of age undergoing KD hospitalization between 1997 and 1999, Belay et al. reported a median hospitalization cost of US$6,169, which was higher than the cost of hospitalization for RS viral bronchiolitis, diarrhea, and rotavirus infection. In a relatively recent report, Ghimire et al. studied the length of stay and cost of inpatient hospitalization in 10,486 cases from 2009 to 2012 in the United States. The length of stay was 4.10 days for weekend hospitalization and 3.72 days for weekday hospitalization, compared to 5.95 days for patients with CAA. It was longer than 3.76 days for cases without CAA. Hospitalization costs averaged US$31,294 for weekend hospitalization and US$34,303 for weekday hospitalization. Meanwhile, the hospitalization cost was US$56,089 for patients with CAA and US$31,178 for patients without CAA. Differences in hospital length of stay and costs were observed by race and type of health insurance, suggesting challenges inherent in the American health care system.
Studies comparing the medical costs of multiple treatment strategies have also been carried out. Klassen et al. compared three treatment strategies in Canada: ASA only, low-dose IVIG, and high-dose IVIG. They found that high-dose IVIG resulted in the least incidence of CAA formation, and the expected cost of high-dose IVIG values was estimated to be the cheapest. Arj-ong et al. conducted a cost-benefit analysis of IVIG in Thailand and estimated that the IVIG group was more expensive in the acute phase, but less expensive in the long term.
According to a health economic study by Sato et al. in Japan, among 145 KD patients with a Harada score of 4 or higher admitted between 1991 and 1995, 72 patients receiving a single dose of IVIG 2 g/kg had a reduced incidence of CAA compared to the 73 patients receiving 400 mg/kg/day for 5 days, as well as significantly lower hospital stays and medical costs. Ogata et al. compared 27 patients with KD refractory to IVIG between April 2004 and May 2007, and found no significant difference in the incidence of CAA or the length of additional hospital stay between IVIG additional therapy (14 patients) and IVMP therapy (13 patients). The mean medical costs were reported to be significantly lower in the IVMP therapy group, at JP¥918,300 and JP¥290,610, respectively. In contrast, another study found no significant difference in health care costs due to the need for additional treatment after IVMP.
Recently, Okubo et al. used diagnosis procedure combination (DPC) data from July 2010 to March 2015 for 24,517 inpatients, categorizing hospitals based on early steroid use before and after the introduction of acute care guidelines, and compared trends in medical costs. The 17 hospitals that started the early use of steroids after the introduction of the guidelines showed a decrease in inpatient hospitalization costs from JP¥289,294 to JP¥266,495. Initial combination therapy for patients who are predicted non-responders to IVIG may help reduce health care costs by reducing the rate of additional IVIG administration and CAA complications.
These results indicate that although the acute treatment of KD is expensive, appropriate and efficient treatment based on medical guidelines can reduce medical costs in the acute phase of the disease. Given the limited research on long-term cost-effectiveness, however, future research should not only demonstrate the efficacy of treatment but also its value by studying the impact of reducing the incidence of CAA with appropriate therapeutic interventions for KD on patient quality of life and prognosis as well as the impact on long-term health care costs.
I. Immunoglobulins
1. Purpose
Currently, the early initiation of IVIG, namely, the high-dose administration of intact-type immunoglobulin, has been recognized as the most reliable anti-inflammatory treatment in acute KD, and can prevent the complication of CAA.40–43)
2. Mechanism of Action
The mechanism is speculative because the cause of the disease is unknown. It is considered to be due to modulation of immune abnormalities by the various pathways shown in Table 2.44–47)
Table 2 Immunomodulatory effects of intravenous immunoglobulin30–33)| 1. Anti-inflammatory action with Fc receptor–mediated neutralizing antibody |
| ·Reduction of complement-mediated disruptive effects |
| ·Reduction of immune complex–mediated inflammation |
| ·Induction of anti-inflammatory cytokines |
| ·Inhibition of vascular endothelial cell activation |
| ·Neutralization of bacterial toxins and super antigens |
| ·Modulation of matrix metalloproteinase |
| 2. Action of immune cells and antibodies |
| ·Regulation of T cell production of cytokines and chemokines |
| ·Neutralization of T cell super-antigen |
| ·Inhibition of dendritic cell differentiation and maturation |
| ·Regulation of inflammatory cytokine and chemokine production |
| ·Inhibition of autoantibody production against vascular endothelial cells |
| ·Enhanced phagocytosis of neutrophils and macrophages (opsonin effect) |
| ·Suppression of mRNA in inflammation-related genes (S100) expressed on monocytes |
3. Indications (Fig. 2)
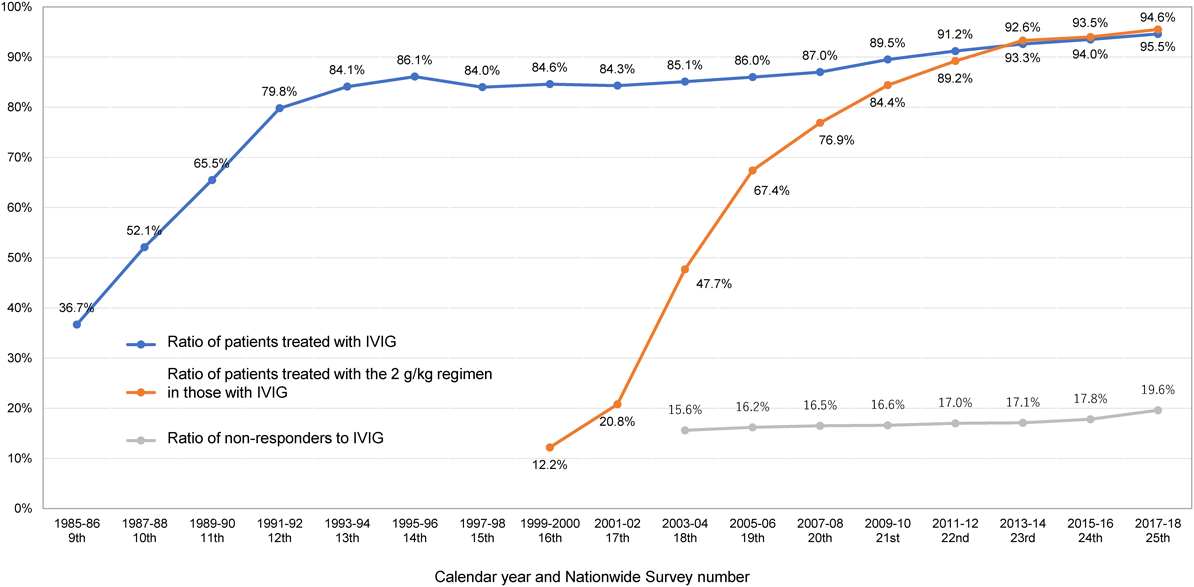
Almost all febrile acute KD patients with possible risk for the complication of CAA who have typical manifestations fulfilling the diagnostic guidelines (6th revised edition)14) have IVIG indication. In addition, IVIG is recommended to be initiated as early as possible in patients diagnosed with incomplete KD according to the diagnostic guidelines (those who have four principal symptoms without CAA including dilatation or three or fewer principal symptoms after exclusion of other diseases), because they are sometimes accompanied by CAA.15) As shown in Fig. 2, IVIG has become widespread throughout Japan since the late 1980s, and is currently administered to most KD patients. The proportion of IVIG administration was 94.6% in the results of the 25th Nationwide Survey of KD.1) A few patients with mild symptoms or spontaneous resolution of fever did not, however, receive IVIG in consideration of the severity criteria for each institution.48)
4. Dosage and Usage
(1) Timing of Administration
IVIG should be started by the 7th day of illness. It is particularly important that treatment effectively relieves fever and inflammation by the 9th day, before the onset of CAA formation. Specifically, the aim is the earlier reduction of fever and inflammatory markers of vasculitis such as C-reactive protein (CRP).
In the diagnostic guidelines for KD (6th revised edition),14) the criterion of days was removed for determining fever as a principal sign, reflecting the increase in cases diagnosed before 5 days of illness. It was reported that, in the patients who received treatment on and before the 5th day, the time from the start of treatment to fever resolution was slightly longer than that in those who received treatment on the 6th to 9th day, but the overall duration of fever was shorter, the rate of recurrent fever as well as additional IVIG administration and hospitalization days were similar, and the prevalence of CAA complication was lower in the first year after disease onset.49)
The results of the 25th Nationwide Survey of KD1) showed that IVIG was initiated on the 5th (34.8%), 4th (25.2%), 6th (16.4%), 3rd (9.1%), 7th (7.3%), 8th (2.9%), 2nd (1.6%), 9th (1.2%), and 1st (0.1%) day of illness in descending order.
(2) Dosage (Fig. 2)
Acute IVIG doses in the acute phase are usually either of the following in the package inserts.
- i) 2 g/kg/day (single dose regimen)
- ii) 200 to 400 mg/kg/day for 3 to 5 days (divided dose regimen)
The single dose regimen, 2 g/kg/day, has significantly lower incidence of CAA, earlier reduction of inflammatory markers, and higher effect of defervescence in comparison with the divided dose regimen.42, 43) As shown in the graph of the IVIG 2 g/kg/day implementation rate in Fig. 2, the single dose regimen has been used in almost 90 to 95% of cases over the past 10 years. The modified single dose regimen, 1 g/kg/day×1 day or 2 consecutive days, described in the previous guidelines3) was used as a transition phase to a single dose of 2 g/kg/day, but is now exceptional and not listed in the package insert. Although high medical costs have resulted in there being no consensus on the dosage for older and heavier children, they are often treated with a single dose of 2 g/kg/day.
Although the infusion rate varies among preparations, the single dose of IVIG, 2 g/kg, is generally intravenously administered over 12 to 24 h in Japan and 10 to 12 h in the United States. During IVIG administration, physicians should pay close attention to the development of heart failure and worsening of cardiac function due to rapid volume loading, and should be careful not to administer it too fast. The use of divided doses appears to be rare today.
(3) Types and Usage
Currently, there are four types of intact immunoglobulin preparations approved by the pharmaceutical authorities in Japan: 2 polyethylene glycol (PEG)–treated preparations, a sulfonated preparation, and a pH 4 acid–treated preparation. There was no apparent difference in efficacy among these preparations. Table 3 provides details based on the package insert.
Table 3 Formulation types of intravenous immunoglobulin (IVIG), and the dosage and usage for Kawasaki disease (KD)| Product name | Kenketsu Venilon-I (for intravenous use) | Kenketsu Glovenin-I (for intravenous use) | Kenketsu Venoglobulin IH 5% (for intravenous use) | Kenketsu Venoglobulin IH 10% (for intravenous use) | Kenketsu Polyglobin N 5% (for intravenous use) | Kenketsu Polyglobin N 10% (for intravenous use) |
|---|
| Generic name | Freeze-dried sulfonated human normal immunoglobulin | Freeze-dried polyethylene glycol-treated human normal immunoglobulin | Polyethylene glycol-treated human normal immunoglobulin | Polyethylene glycol-treated human normal immunoglobulin | pH 4-treated acidic human normal immunoglobulin | pH 4-treated acidic human normal immunoglobulin |
| Company (manufacturer/distributor) | K M Biologics—Teijin Pharma Limited | Nihon Pharmaceutical—Takeda Pharmaceutical | Japan Blood Products Organaization | Japan Blood Products Organaization | Japan Blood Products Organaization | Japan Blood Products Organaization |
| Form of medication | Freeze-dried preparation | Freeze-dried preparation | Liquid medication | Liquid medication | Liquid medication | Liquid medication |
| Constituents (in 2.5 g of product) | Sulfonated human immunoglobulin G 2,500 mg
Glycin 975 mg
Human plasma albumin 125 mg
D-mannitol 450 mg
Sodium chloride 500 mg | Polyethylene glycol-treated human immunoglobulin G 2,500 mg
D-mannitol 750 mg
Glycin 225 mgSodium chloride 450 mg | Human immunoglobulin G 2,500 mg
D-sorbitol 2,370 mg
Sodium hydoxide, suitable amount
Hydrochloric acid, suitable amount | Human immunoglobulin G 2,500 mg
Glycin 380 mg
Sodium hydoxide, suitable amount
Hydrochloric acid, suitable amount | Human immunoglobulin G 2,500 mg
Maltose hydrate 5,000 mg
Sodium hydoxide Suitable amount
Hydrochloric acid Suitable amount | Human immunoglobulin G 2,500 mg
Glycin 750 mg
Sodium hydoxide Suitable amount
Hydrochloric acid Suitable amount |
| Dosage and usage | Normally given as sulfonated human immunoglobulin G by direct or drip intravenous infusion, at 200 mg (4 mL)/kg bodyweight/day over a 5 day period. Alternatively, a single dose of 2,000 mg (40 mL)/kg bodyweight may be given intravenously. In addition, in the case of 5 day treatment, this dose may be adjusted according to patient age and condition. In the case of one-time treatment, the dose may be similarly reduced as required. | Normally given as human immunoglobulin G by direct or drip intravenous infusion, at 200 mg (4 mL)/kg bodyweight/day over a 5 day period. Alternatively,a single dose of 2,000 mg (40 mL)/kg bodyweight may be given intravenously. In addition,in the case of 5 day treatment, this dose may be adjusted according to patient age and condition. In the case of one-time treatment, the dose may be similarly reduced as required. | Normally given as human immunoglobulin G by direct or drip intravenous infusion, at 400 mg (8 mL)/kg bodyweight/day over a 5 day period. Alternatively,a single dose of 2,000 mg (40 mL)/kg bodyweight may be given intravenously. The dose may be reduced according to patient age and condition. | Normally given as human immunoglobulin G by direct or drip intravenous infusion, at 400 mg (4 mL)/kg bodyweight/day over a 5 day period. Alternatively,a single dose of 2,000 mg (20 mL)/kg bodyweight may be given intravenously. The dose may be reduced according to patient age and condition. | Normally given as human immunoglobulin G by direct or drip intravenous infusion, at 200 mg (4 mL)/kg bodyweight/day over a 5 day period. Alternatively,a single dose of 2,000 mg (40 mL)/kg bodyweight may be given intravenously. In addition, in the case of 5 day treatment, this dose may be adjusted according to patient age and condition. In the case of one-time treatment, the dose may be similarly reduced as required. | Normally given as human immunoglobulin G by direct or drip intravenous infusion, at 200 mg (2 mL)/kg bodyweight/day over a 5 day period. Alternatively,a single dose of 2,000 mg (20 mL)/kg bodyweight may be given intravenously. In addition, in the case of 5 day treatment, this dose may be adjusted according to patient age and condition. In the case of one-time treatment, the dose may be similarly reduced as required. |
| Precautions regarding dosage and usage | A sudden drop in blood pressure may occur if the medication is given too rapidly (special care is required when the patient's blood contain little or no endogenous γ-globulin). | |
| Treatment speed: 1) On the 1st day, the drug should be delivered at a rate of 0.01 to 0.02 mL/kg/min during the initial 30 min. If no side effects or other abnormalities are observed, the administration speed may be gradually increased to 0.03 to 0.06 mL/kg/min. On the 2nd day and later, the dose is administered at the rate tolerated on the previous day.2) In the case of a one-time treatment of 2,000 mg (40 mL)/kg to KD patients, the administration rate should be basically the same as in 1), and the infusion period is generally 12 h and over. | Treatment speed: Because there is a possibillty of shock or other serious side effects during the initial hour of treatment on the 1st day, and also when the treatment speed is increased, the patient must be carefully monitored during these times.1) On the 1st day, the treatment speed should be 0.01 mL/kg/min during the initial hour. When the absence of side effects and other problems has been confirmed, the speed may be gradually increased. However, it should not exceed 0.06 mL/kg/min. On the 2nd day and later, the dose is administered at the rate tolerated on the previous day.2) In the case of one-time treatment of 2,000 mg (40 mL)/kg to KD patients, the administration rate should be basically the same as in 1), and the infusion period is 12 h and over, with careful attention paid to a sudden increase in circulatory blood volume. | Treatment speed: Because there is a possibillty of shock or other serious side effects during the initial hour of treatment on the 1st day, and also when treatment speed is increased, the patient must be carefully monitored during these times.1) On the 1st day, the treatment speed should be 0.01 mL/kg/min during the initial hour. When the absence of side effects and other problems has been confirmed, the rate may be gradually increased. However, it should not exceed 0.06 mL/kg/min. On the 2nd day and later, the dose is administered at the rate tolerated on the previous day.2) In the cases of one-time treatment of 2,000 mg (40 mL)/kg to KD patients, the administration rate should be basically the same as in 1), and the infusion period is 12 h and over, with careful attention paid to sudden increase in circulatory blood volume. | Treatment speed: Because there is a possibillty of shock or other serious side effects during the initial hour of treatment on the 1st day, and also when treatment speed is increased, the patient must be carefully monitored during these times.1) On the 1st day, the treatment speed should be 0.01 mL/kg/min during the inital hour. When the absence of side effects and other problems has been confirmed, the rate may be gradually increased. However, it should not exceed 0.06 mL/kg/min. On the 2nd day and later, the dose is administered at the rate tolerated on the previous day.2) In the case of one-time treatment of 2,000 mg (20 mL)/kg to KD patients, the administration rate should be basically the same as in 1), and the infusion period is 6 h and over, with careful attention paid to sudden increase in circulatory blood volume. | Treatment speed: 1) On the 1st day, the drug should be delivered at a rate of 0.01 to 0.02 mL/kg/min during the initial 30 min. If no side effects or other abnormalities are observed, the rate may be gradually increased to 0.03 to 0.06 mL/kg/min. On the 2nd day and later, the dose is administered at the rate tolerated on the previous day.2) In the case of one-time treatment of 2,000 mg (40 mL)/kg to KD patients, the administration rate should be basically the same as in 1), and the infusion period is 12 hs and over. | Treatment speed:1) On the 1st day, the drug should be delivered at a rate of 0.01 to 0.02 mL/kg/min during the initial 30 min. If no side effects or other abnormalities are observed, the rate may be gradually increased to 0.03 to 0.06 mL/kg/min. On the 2nd day and later, the dose is administered at the rate tolerated on the previous day.2) In the case of one-time treatment of 2,000 mg (20 mL)/kg to KD patients, the administration rate should be basically the same as in 1), and the infusion period is 6 h and over. |
The main differences between the preparations are as follows:
- i) The sulfonated formulation is a 5% lyophilized preparation containing a small amount of serum albumin, and the sodium concentration is 154 mEq/L, the same as saline.
- ii) PEG products are available in 5% lyophilized and liquid formulations (5% and 10%). The former has a sodium concentration of 154 mEq/L. Because the latter is kept refrigerated, care should be taken to bring it to room temperature before administration and to ensure that the drug does not leak outside the vessel when administered intravenously.
- iii) The pH 4 acid–treated preparations (5% and 10%) should be brought to room temperature before administration because they are liquefied and stored in a cold place. In addition, because of the addition of maltose in the 5% formulation, caution should be taken not to use blood glucose dehydrogenase assays, which can be affected during post-dose blood glucose monitoring.
- iv) According to a report on the 10% formulation, which is beginning to be used, the dosage volume is halved, the administration time is reduced to half, and the antipyretic effect may be faster than that of the 5% formulation50); the differences between these formulations need to be examined in the future. In the present guidelines, the determination of non-responders to IVIG is made 24 to 36 h after the end of IVIG whereas this was previously done 24 h after the end of IVIG.19)
Because side effects such as anaphylaxis are likely to occur within the first hour of administration or at a rapid rate of administration, special attention is needed during the initial 30 min to 1 h following the start of administration. If there are no signs of abnormality, the remaining 2 g/kg of the 5% formulation is administered over 12 to 24 h and the 10% formulation over 6 to 12 h.
(4) Additional IVIG for Non-responders to IVIG (Fig. 2)
As shown in Fig. 2, after standard treatment with IVIG and ASA, approximately 15 to 20% (19.7% in the 25th Nationwide Survey of KD1)) of patients have insufficient antipyretic effect (non-responders to IVIG), and the rate has slightly increased since 15 years ago, when it was in the 15% range. These patients are generally treated with re-administration of IVIG. According to the results of the 25th Nationwide Survey of KD, among additional treatments to 6,061 non-responders to initial IVIG, the majority (91.1%) of them were treated with IVIG again.1) Re-administration of IVIG was reported to improve symptoms in approximately half of non-responders.51)
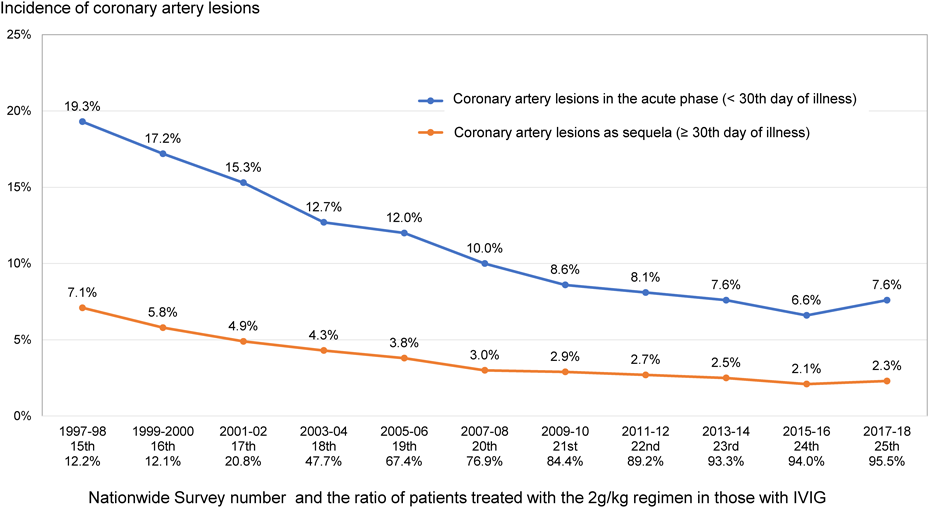
5. Usefulness (Fig. 3)
IVIG is currently the safest and most reliable treatment, and its efficacy is recognized worldwide and described in many textbooks and guidelines.40–43) IVIG was established in two double-blind randomized controlled trials by Newburger et al.,41, 42) following a randomized, open-label, controlled trial by Furusho et al.40) According to a systematic review and meta-analysis of the Cochrane Library,43) IVIG significantly reduced the incidence of CAA at 30 days of illness compared with placebo (combined odds ratio [OR] 0.74, 95% confidence interval [CI] 0.61–0.90). In addition, a single 2 g/kg dose was shown to reduce the incidence of CAA (combined OR 4.47, 95% CI 1.55–12.86) compared with divided doses of 400 mg/kg/day for 5 days.
The most important indicator of the benefit of IVIG for KD is the incidence of cardiac complications including CAA, which has decreased with the increase in the number of patients receiving a single dose of 2 g/kg/day, as shown in Fig. 3. In the 25th Nationwide Survey on KD,1) the rate of coronary artery lesion in the acute phase within the 30th day of illness was 7.6%, and the percentage of coronary sequelae remaining after the 31st day was 2.3%. In addition to coronary arteries, valvular lesions also occurred in 1 to 2% of patients within the 30th day and in less than 0.5% after the 31st day. The incidence of coronary artery complications was reduced to roughly one-third of that in 1997 to 1998 when a single dose 2 g/kg regimen was rarely used.
6. Side Effects (Table 4)52, 53)
There have been no reports of viral infections with intact-type immunoglobulins in Japan. Donor blood is screened for HBs antigen, anti-HCV, anti-HIV-1, anti-HIV-2, and anti-HTLV-I antibodies, and for ALT levels. In addition, the pooled plasma is tested for nucleic acid amplification for HAV, HBV, HCV, HIV, and human parvovirus B19, and only compatible plasma is used. The possibility of infection due to contamination with abnormal prions and viruses below the detection limit of the nucleic acid amplification test, such as human parvovirus B19, cannot be ruled out during the current manufacturing process of each formulation, but there have been no reports of post-dose infection.
Table 4 Common side effects of IVIG52, 53) | High Incidence | Rare |
|---|
| General | Fatigue, fever, facial redness, chills | Anaphylaxis |
| Systemic side effects | Anorexia, myalgia, arthralgia, joint swelling | Common cold symptoms, anaphylaxis, eyelid edema |
| Neurological | Headache, migraine, dizziness | Aseptic meningitis, weakness, dysesthesia |
| Respiratory | Shortness of breath, cough, bronchoconstriction | Pleural effusion, transfusion-related lung injury, pulmonary edema |
| Cardiovascular | Low blood pressure, high blood pressure, chest pain | Arrhythmia, myocardial infarction |
| Gastrointestinal | Anorexia, nausea, vomiting, abdominal pain, diarrhea | Taste impairment |
| Renal | | Tubular disorders, renal failure |
| Dermatological | Urticaria, erythema, papules, and pruritus | Urticaria, erythema multiforme exudative |
| Hematological | Hemolysis | Thromboembolism, hyperviscosity syndrome,leukopenia |
Although infrequent, the side effects reported to date that require close observation include chills/shivers, shock (cyanosis, hypotension), anaphylactic reactions, aseptic meningitis,54) hemolytic anemia,55) hepatic dysfunction, jaundice, acute renal failure, thrombocytopenia, and pulmonary edema. It is particularly important to pay attention to chills, shivering, unconsciousness, restlessness, tremor, cyanosis, hypotension, and shock immediately after starting intravenous infusion and increasing the administration rate. Being aware of potential myocardial damage and heart failure in the acute phase, physicians should watch for rapid increases in circulating blood volume and changes in vital signs during the intravenous infusion. Other precautions include careful administration in the following cases:
- i) Patients with IgA deficiency: in patients with anti-IgA antibodies, hypersensitivity reaction may occur.
- ii) Patients with renal impairment: renal function may be worsened.
- iii) Patients with cerebral or cardiovascular disorders or a history of such disorders: the increase in blood viscosity due to large doses may cause thromboembolism, such as cerebral or myocardial infarction.
- iv) Patients at high risk of thromboembolism: thromboembolism may occur due to an increase in blood viscosity caused by large doses.
- v) Patients with hemolytic or exsanguinated anemia, immunodeficiency, or immunosuppression: the possibility of human parvovirus B19 infection cannot be ruled out. Infection can cause severe systemic symptoms with fever and sudden anemia and persistent anemia.
- vi) Patients with impaired cardiac function: Large doses may cause or worsen heart failure.
According to a large-scale post-marketing study for 7,259 KD patients who received IVIG, there were 697 cases in 484 patients of side effects (9.6%), and among these, 78 cases in 68 patients (1.1%) of serious side effects.56) Although IVIG is a safe treatment with rare side effects, it is essential to obtain the consent of patients and their families at the time of use because it is a blood product.
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Initial treatment with IVIG | I | A |
| Additional treatment with IVIG for non-responders to IVIG | I | C |
II. Steroids (Table 5)
Table 5 Treatments other than intavenous immunoglobulin (IVIG) for acute Kawasaki disease| General name | Mechanisms | Treatment route, dosage, and usage | Main side effects | Precautions |
|---|
| Prednisolone | Inhibition of the gene transcription of inflammatory proteins via cytoplasmic steroid receptors. | In the febrile phase, 2 mg/kg/day is given intravenously in 3 divided doses. After defervescence and improvement in the patient’s general condition, PSL can be given orally. In the RAISE Study, the patient was continued for 5 days on the same dosage in three divided doses of 2 mg/kg per day after CRP normalized. Thereafter, if fever does not recur, the dosage of PSL is decreased to 1 mg/kg per day in two divided doses on the subsequent 5 days and then a single dose of 0.5 mg/kg per day on the final 5 days. | Viral infection (a few percent), moon face (in most cases), hypothermia immediately after defervescence (a few percent), positive fecal occult blood (approximately 1%), hyperlipidemia (a few 10%), and neutrophil-predominant leukocytosis (almost all cases). | Blood tests and echocardiography should be carefully monitored because it may be difficult to detect relapse due to changes in temperature and CRP level. If relapse is suspected, appropriate intervention should be considered. The most common periods for relapse are 4 to 5 days after the start and after the dose reduction to 1 mg/kg/day. |
| Methylprednisolone | In addition to the above, the suppression of immune cells and pro-inflammatory cytokines by non-genomic effects such as changes in cell membrane function. | A dose of 30 mg/kg, once a day, for 1 to 3 days. Some reports suggest additional prednisolone after ending methylprednisolone. | Sinus bradycardia (6–82%), hypertension (10–91%), hyperglycemia (6–55%), hypothermia (6–9%), etc. In rare cases, patients may develop infections, gastrointestinal ulcers, mental disorders, femoral head necrosis, and suppressed adrenal function. | Vital signs including electrocardiogram, body temperature, and blood pressure should be continuously monitored. |
| Cyclosporine A | Suppression of cytokine production such as IL-2 by inhibiting nuclear factor of activated T cells. | Start on 2 divided oral doses of 5 mg/kg/day (before meals in the morning and evening). Adjustment for target trough level, 60–200 ng/mL. | General adverse reactions include increased blood pressure, nausea and vomiting, shivering, hyperglycemia, hyperuricemia, hyperlipidemia (1 – 5%) etc. | Subclinical hyperkalemia (with lower values in plasma than in serum). In addition, there have been some reports of hypomagnesemia, but no adverse events such as arrhythmias have been observed to date. |
| Infliximab | Neutralization of soluble TNF-α biological activity, damaging membrane-bound TNF-α-expressing cells with complement- and antibody-dependent cell damage, and dissociation of TNF-α bound to TNF-αreceptors. | Intravenous drip infusion of 5 mg/kg (may only be given once). | Eruption (2.7%), viral/bacterial infection (2.1%), infusion reaction (1.4%), neutropenia (0.3%), liver dysfunction (0.3%), etc. | Imaging and laboratory tests to rule out tuberculosis and hepatitis are recommended prior to administration. After administration, vital signs should be monitored carefully with ambulatory electrocardiogram. |
| Ulinastatin | Inhibits elastase release from neutrophils and platelets, rendering it inactive after release. | Intravenous drip of 5,000 units/kg, 3 – 6 times a day, for several days, not over 300,000 units/day. | Anaphylaxis (incidence unknown), liver dysfunction (0.5%), leukopenia (0.2%), allergic symptoms such as exanthema and pruritus (0.1%), diarrhea (0.1%), angiodynia (0.1%), transient elevation of AST and ALT, eosinophilia, vascular pain at injection site etc. | Avoid mixing with IVIG in the treatment route. |
| Plasma exchange | Mechanical removal of inflammatory cytokines. | Displacing solution set at 5% albumin; 1 –1.5 times as the patient’s circulating plasma volume is exchanged, usually given for 3 continuous days (upper limit: 6 days). | Hypotension, hypovolemia, shock, anaphylactoid reactions, hypocalcemia, fever/coldness/shivering, nausea/vomiting, coagulopathies, thrombosis and damage at time of catheter insertion etc. | |
A. Prednisolone (PSL)
1. Purpose
The primary purpose of PSL therapy is to take advantage of its powerful anti-inflammatory effects. PSL can quickly resolve KD vasculitis and suppress the potential risk for the remodeling of coronary arteries.
2. Mechanism of Action
PSL is the most widely used synthetic corticosteroid hormone, and its glucocorticoid action is stronger than that of cortisol. Through cytoplasmic steroid receptors, PSL inhibits the gene transcription of inflammatory proteins, promotes the gene transcription of anti-inflammatory proteins, and thereby has strong anti-inflammatory effects. PSL also suppresses vasculitis by inhibiting the production of inflammatory cytokines (e.g., tumor necrosis factor [TNF]-α, interleukin [IL]-6, IL-8, IL-8, G-CSF), chemokines, and cell adhesion molecules) and promoting anti-inflammatory proteins such as lipocortin.57)
3. Indications
PSL has insurance coverage in the acute phase of KD (in severe cases at risk of coronary artery lesions). Many institutions use PSL in severe KD patients whose Kobayashi score predicting IVIG non-response is 5 points or more in combination with a single dose of IVIG 2 g/kg with a medium dose of ASA based on the protocol of the RAISE study.4, 20)
4. Dosage and Usage
When PSL is used in combination with IVIG as the initial treatment, the dosage and usage are generally in accordance with the RAISE study.4) For non-responders to initial IVIG, the regimen for second-line PSL should, in principle, involve the same method as specified for first-line PSL therapy. In the febrile phase, PSL 2 mg/kg/day is given intravenously in three divided doses. After defervescence and improvement in the patient’s general condition, PSL can be given orally. In the RAISE study, the same dosage is continued for 5 days in three divided doses after CRP normalizes. Thereafter, if fever does not recur, the dosage of PSL is decreased to 1 mg/kg/day in two divided doses on the subsequent 5 days, and then to a single dose of 0.5 mg/kg/day on the final 5 days. If fever recurs after dose reduction, additional treatment should be considered, including an increase in PSL dose, IVIG retreatment, or other treatments.
During the administration of PSL, it may be difficult to determine relapse by changes in inflammatory markers such as temperature and CRP level. Therefore, more frequent blood tests and echocardiograms should be done during PSL treatment, and appropriate interventions should be implemented if relapse is suspected. The most common periods for relapse are 4 to 5 days after the start of PSL and after the dose reduction to 1 mg/kg/day.
5. Usefulness
In the old days, PSL was widely used in KD patients. A case-control study showed that fatal cases were more frequently treated with PSL, however.58) In addition, a retrospective study found that PSL had a detrimental effect on the incidence of CAA when used as mono-therapy.59) Finally, a prospective randomized controlled trial of three groups (receiving either aspirin, flurbiprofen, or PSL plus dipyridamole) did not confirm the efficacy of PSL.60) These results led to PSL being contraindicated for KD in several decades.
In 2006, a randomized controlled trial61) was carried out to assess the efficacy of initial treatment with IVIG plus PSL versus IVIG mono-treatment in preventing CAA in all KD patients. Although the trial did not reach the target patient number due to a low entry rate, they found that initial treatment with IVIG plus PSL significantly reduced the incidence of CAA. PSL was assumed to be more effective in inhibiting CAA formation in patients with severe KD defined by using the Kobayashi score.62) As such, a randomized controlled trial to assess immunoglobulin plus steroid efficacy for KD (RAISE study)4) was carried out. The RAISE study showed that, among severe KD patients with a Kobayashi score ≥5 points, initial treatment with IVIG plus PSL significantly decreased the incidence of CAA and additional treatment-necessary cases compared with IVIG. A systematic review and meta-analysis of the Cochrane Library7) indicated that initial treatment with IVIG plus PSL has moderate evidence to reduce the risk of CAA (combined OR 0.13, 95% CI 0.05–0.32) compared with IVIG. The large prospective cohort study (Post RAISE)5) reported that the initial treatment outcomes with IVIG plus PSL were similar to those of IVIG plus PSL in the RAISE study.
Because no well-designed randomized controlled trials have been conducted, the causal effect of PSL in patients who are non-responders to initial IVIG remains unclear. A retrospective observational study63) suggested that an additional treatment with IVIG plus PSL combination therapy results in a lower risk of CAA formation and a lower need for additional treatment than IVIG or PSL mono-therapy. It should be noted, however, that PSL may not reduce the risk of CAA formation if PSL is administered in the late phase of KD.63, 64)
6. Side Effects
The side effects of PSL in acute KD patients include viral infections (a few %), full moon–like face (majority of cases), hypothermia immediately after defervescence (a few %), positive stool latent blood test (around 1%), hyperlipidemia (dozens of %), and neutrophil-dominant leucocytosis (almost all cases). Generally, these cases improve with appropriate treatment or observation. Bacterial infections such as urinary tract infections and sepsis (less than 1%) require caution, and appropriate antimicrobials should be administered after differentiating them from KD relapse. Although no serious adverse effects have been reported, clinicians must monitor for sinus bradycardia65) and adrenal insufficiency66) caused by PSL administration.
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Initial IVIG plus PSL for predicted non-responders to IVIG | I | A |
| Additional treatment with IVIG plus PSL for non-responders to IVIG | IIa | C |
B. Methylprednisolone Pulse (IVMP)
1. Purpose
IVMP treatment, the high-dose intravenous infusion of methylprednisolone, is administered to rapidly suppress vasculitis based on its powerful and rapid immunosuppressive effect. Among available steroids, methylprednisolone is less likely to disrupt the electrolyte balance. IVMP is widely used in treating severe pediatric illnesses such as rheumatic diseases and kidney diseases. In KD, IVMP is used in treating non-responders to IVIG as an additional rescue therapy or in predicted non-responders to IVIG as an initial adjunctive therapy with IVIG.
2. Mechanism of Action
Steroids bind with glucocorticoid receptors in the cytoplasm and regulate the nuclear expression of proteins such as NF-κB, which produces an anti-inflammatory effect referred to as genomic action.57) When high-dose methylprednisolone is given, the saturation point of these glucocorticoid receptors is greatly exceeded. Thus, mechanisms other than genomic action are thought to contribute to its efficacy. These mechanisms may involve acting through proteins that dissociate from complexes with cytosolic glucocorticoid receptors, membrane-bound glucocorticoid receptors, and functional modification of membrane-bound protein after inter-location of the cell membrane. These non-genomic mechanisms precede genomic action.57, 67)
When IVMP is used in KD patients, the effects are rapid. This suggests that non-genomic mechanisms stimulate immune-cytological activity and suppress inflammatory cytokines. In confirmed or predicted non-responders to IVIG, IVMP reportedly reduced the production of cytokines,68) and transcription at the genetic levels69) is involved in inflammation and CAA development.
3. Indications
IVMP is used as a combined therapy with initial IVIG for predicted non-responders to IVIG or as an additional rescue therapy for non-responders to IVIG, but it is not covered by insurance for KD.
4. Dosage and Usage
In patients with kidney diseases or connective-tissue diseases, the standard dose of methylprednisolone is 20 to 30 mg/kg, infused intravenously once a day over 2 to 3 h for 3 consecutive days.67) The IVMP regimens for KD patients reported in previous studies are as follows: a single dose of 30 mg/kg in combination with initial IVIG for first-line treatment23, 24, 70) or the same dose given once a day for 1 to 3 consecutive days as an additional rescue treatment for non-responders to IVIG.37, 68, 69, 71–73)
5. Usefulness
In a double-blind randomized controlled trial comparing IVIG plus IVMP with IVIG plus placebo, no significant differences were found in terms of duration of fever, incidence of additional treatment, and incidence of CAA.70) A post hoc subgroup analysis of patients who required additional treatment, however, found that the incidence of CAA was significantly lower among those who had received IVIG plus IVMP. Some studies have reported that predicted non-responders to IVIG on the basis of Sano score (historical control study)23) or Egami score (unblinded randomized controlled trial)24) who received IVIG plus IVMP as the first-line treatment had earlier defervescence and a significantly lower incidence of CAA than those who received IVIG alone. In a prospective cohort study of predicted non-responders to IVIG defined by Egami score,74) a treatment protocol of initial IVIG plus IVMP for the first-line treatment, additional IVIG for the second line, and IFX or PE for the third line was effective in preventing CAA. A meta-analysis showed that a combination of steroid including PSL or IVMP with IVIG as an initial treatment significantly reduced the incidence rate of CAA compared to IVIG alone.6, 7) According to a systematic review and a meta-analysis by the Cochrane Library,7) the efficacy of IVMP (one-off steroid use) in preventing CAA development had no statistical significance in sensitivity analysis (combined OR 0.56, 95% CI 0.29–1.08).
Several studies reported that the incidence of CAA was similar between non-responders to IVIG treated with IVMP as an additional rescue therapy and patients who received an additional IVIG treatment.37, 38, 68, 71) Since the half-life of IVMP is only 3 h,67) the IVMP regimen followed by additional PSL (1–2 mg/kg/day at the starting dose, tapered gradually over 1–3 weeks) was used in some studies.72, 73)
6. Side Effects
The reported side effects of IVMP treatment for KD patients include sinus bradycardia (6–82%), hypertension (10–91%), hyperglycemia (6–55%), and hypothermia (6–9%).72, 75) As such, vital signs must be carefully monitored during IVMP, including monitoring of electrocardiogram and blood pressure. To avoid the development of gastrointestinal ulcer, patients can be given H2 blockers and/or other antacid agents, and additional heparin can also be given as prophylaxis for thrombosis.72, 73) The necessity of these medications has not been proven, however. Steroids may lead to other side effects, such as infection, abnormal mental function, osteonecrosis of the femoral head, secondary adrenocortical insufficiency, etc. Since IVMP involves a short-term administration, however, these side effects are less likely to occur.
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Initial IVIG plus IVMP for predicted non-responders to IVIG | IIa | B |
| IVMP as an additional rescue treatment for non-responders to IVIG | IIa | B |
III. Immunosuppressants (Table 5)
A. Cyclosporin A (CsA)
1. Purpose
Since 2008, it has been reported that multiple genes associated with the nuclear factor of the activated T cells (Ca2+/NFAT) pathway in immunocytes, including T cells, contribute to KD susceptibility and CAA development.76–78) This suggests that activation of the Ca2+/NFAT pathway plays an important role in KD vasculitis. CsA is a drug that inhibits the Ca2+/NFAT pathway, thereby inhibiting the vasculitis and vessel wall destruction that characterizes KD.
2. Mechanism of Action
CsA binds to calcineurin, which plays an important role in signal transduction during the activation of immunocytes, including T cells, and inhibits nuclear transport through dephosphorylation of the transcription factor NFAT. In this way, production of inflammatory cytokines such as IL-2 is inhibited.79)
3. Indications
CsA is indicated for KD patients in the acute phase who are seriously ill and at risk of CAA, and is used in combination with initial IVIG for predicted non-responders to IVIG or as additional treatment for non-responders to IVIG. Health insurance approval for CsA as an oral solution was obtained in February 2020, for KD, but the capsule and intravenous preparations remain off-label.
4. Dosage and Usage
CsA combined with IVIG is used as an initial intensified therapy for patients whose risk scores predict that they will be non-responders to IVIG. CsA in liquid form is usually administered at 5 mg/kg/day orally, divided into two doses, before meals in the morning and evening and, in principle, for 5 days.11) Plasma trough levels before dosing on the 3rd day are measured, and if confirmed to be outside the optimal range of 60 to 200 ng/mL, the CsA dose can be changed. Administration before meals is recommended to ensure stability of absorption.
For additional treatment of non-responders to IVIG, CsA is administered orally in liquid form at 5 mg/kg/day divided into two daily doses.80, 81) If an effect becomes evident within 5 days after the start of CsA, the dose can be reduced or stopped if CRP negative conversion is observed, or otherwise within 10 to 14 days.80, 81) Several reports have documented the intravenous use of CsA, for example, infusion at 3 to 4 mg/kg/day divided into two daily doses, or continuous infusion at 3 mg/kg/day, followed by a switch to oral CsA.81, 82)
5. Usefulness
A randomized controlled trial (KAICA Trial) of initial treatment for predicted non-responders to IVIG examined whether one group treated with CsA added to IVIG plus ASA had a lower risk of CAA formation than a group receiving standard therapy with IVIG plus ASA. They showed that the risk of CAA formation was significantly lower in the CsA added to standard treatment group (risk ratio 0.46, 95% CI 0.25–0.86).11)
Observational studies of CsA as a third-line therapy for non-responders to IVIG in Japan and other countries reported that defervescence was obtained within 5 days after the start of CsA treatment and that inflammatory findings such as the CRP level were improved in many cases.80, 81) Non-responders to CsA were present, however, and a third IVIG course was often effective in such patients.80) There has been limited experience of CsA treatment in patients less than 4 months old.83)
6. Side Effects
Although there have been no reports of serious side effects related to CsA use in KD to date, careful attention is needed because rare cases may have gone undetected. Asymptomatic hyperkalemia is detected in serum specimens in approximately 40% of patients. As plasma specimens do not exhibit it, pseudo-hyperkalemia may be present.80) Although hypomagnesemia was reported,81) there have been no reports of arrhythmia due to electrolyte abnormality. In addition, hypertrichosis (with long-term use) has been reported as a side effect with a frequency of 5% or more, and elevated blood pressure, nausea, vomiting, and tremor have been reported in between 1% and 5% of patients. Attention needs to be paid to the combined use of macrolide-based antibiotics that are metabolized with CYP3A4, because CsA is also metabolized by CYP3A4, leading to a possible increase in the CsA blood level. It has been reported that CsA prolongs the disappearance of statins from blood when administered in combination.
When CsA is used for the treatment of KD, there is no clear evidence regarding the safety period after live vaccine inoculation. Physicians need to pay attention to the occurrence of infection and administer CsA after careful consideration of individual risks and benefits.
B. Methotrexate (MTX)
Some reports from other countries indicated that low-dose methotrexate (MTX) was effective for inhibiting vasculitis in non-responders to IVIG.84, 85) The suspected mechanisms of action are as follows: i) suppression of an enzyme relating to purine metabolism, ii) suppression of T-cell activation, iii) selective B-cell suppression, and iv) suppression of methyltransferase activity. Details are lacking, however, and MTX is used off-label for KD in Japan. MTX is administered orally at 10 mg/m2 (up to 16 mg) once a week until defervescence is observed. To date, there have been no randomized controlled trials, and all conventional studies have been retrospective. In most cases, fever subsided significantly within 24 h after low-dose MTX administration, and CRP levels decreased significantly in 1 week.85) Although side effects (leukopenia, liver dysfunction, ulcerative stomatitis, etc.) associated with the standard dose of MTX are rare with low-dose administration, nausea and vomiting may sometimes become a problem.
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Initial treatment with IVIG plus CsA for predicted non-responders to IVIG | IIa | B |
| Additional treatment for non-responders to IVIG | IIb | C |
IV. Biologics (Table 5)
A. Infliximab (IFX)
1. Purpose
IFX inhibits the inflammatory pathways and suppresses vasculitis by specifically blocking the action of TNF-α, mainly in non-responders to IVIG and severe cases.
2. Mechanism of Action86)
IFX is an anti-TNF-α monoclonal antibody that contains approximately 25% mouse protein per molecule. As such, anti-chimeric antibodies (neutralizing antibodies) appear in approximately 40% of cases, and chronic administration may result in reduced efficacy and allergic reactions. The mechanism of action is the binding and neutralization of soluble TNF-α, the dissociation of TNF-α from its receptor, and the suppression of TNF-α by damaging TNF-α-producing cells.
3. Indications
IFX is covered by insurance for KD patients in the acute phase who are refractory to standard treatments, and is mainly used as an additional therapy for non-responders to IVIG.
4. Dosage and Usage
Usually, 5 mg/kg of IFX is intravenously infused after being diluted in 50 to 250 mL of normal saline over 2 h to non-responders to IVIG. The blood concentration reaches its peak 2 h after the end of administration and the half-life is as long as 8 to 10 days. Basically, a single dose of IFX should be principally administered for KD, which is an acute disease, to avoid the side effects associated with frequent administrations. In the United States, a randomized controlled trial of a single 10 mg/kg dose of IFX plus IVIG as the second-line treatment (KIDCARE trial) is underway.87)
5. Usefulness
In Japan, IFX is mainly used as the third-line or later treatment for non-responders to additional IVIG. The incidence of CAA complications is lower if IFX is administered before the 10th day of illness,2) and administration within the 9th day is thus recommended. Approximately 20% of patients do not respond to IFX, and they need to receive prompt additional treatments. IFX as a second-line therapy may also be effective but requires future validation, and should be performed in a professional facility proficient in the use of third-line therapy. The rate of use as a second-line therapy was 2.8% in 434 cases in nationwide surveys conducted between 2005 and 2014,2) and 11.3% in 291 cases in post-marketing surveillance (SAKURA Study) after insurance approval in 2015.88) The mean time to resolution of fever after IFX administration was as short as 16.6 h, and the resolution rate at 48 h after IFX administration ranged from 77.4 to 83.6%.88, 89) In retrospective studies, patients treated with IFX had a higher rate of response to subsequent PE90) and earlier regression of CAA91) than those without IFX treatment. The incidence of long-term CAA complications in patients mainly treated with third-line IFX and fourth-line PE has been reported to be low in several retrospective studies.74, 92)
Randomized trials comparing the second-line treatment with IFX with IVIG for non-responders to the first-line treatment with IVIG reported a higher rate of fever resolution in the IFX group, but no significant difference in the incidence of CAA.8, 93, 94) A systematic review and meta-analysis of TNF-α inhibitors by the Cochrane Library in 201910) found a low level of evidence that the second-line treatment in non-responders to IVIG lowered the risk of unresponsiveness (combined risk ratio 0.46, 95% CI 0.28–0.76) compared with additional IVIG. At present, the risk of CAA complications was not statistically significant (combined risk ratio 0.48, 95% CI 0.11–2.06).
Initial treatment intensification with IVIG plus IFX as the first-line treatment has been reported in a randomized controlled trial,9) but there have been no reports showing a significant reduction in the rate of CAA complications, and thus there is no evidence to recommend its use in all KD cases.
6. Side Effects
The most common side effects of IFX are listed below. Board-certified pediatricians who have learned about IFX through e-learning provided by the pharmaceutical company are required to prescribe IFX to patients with KD.
(1) Infusion Reaction
Since IFX is administered as a single dose in KD, the use of premedication such as antihistamine differs among institutions. Careful observation of the patients and frequent checks of the vital signs are essential after the start of administration. In the SAKURA Study, infusion reactions were observed in 4 of 294 patients (1.4%). In another report of 55 patients at a single center,95) infusion reactions were observed in 1.8% of patients with premedication, one of which was a second-line IFX administration at 7-month intervals due to the recurrence of KD. Particular attention should be paid when IFX is re-administered due to relapse or other reasons.
(2) Exacerbation of Infectious Diseases (Tuberculosis and Viral Hepatitis)
A careful medical interview and chest X-ray are recommended before the administration of IFX to rule out tuberculosis. CT scan of the chest is also an option if necessary. The interferon-gamma release test or tuberculin reaction takes a couple of days to obtain results, and should be performed prior to the anticipated IFX administration. Performing tests for HBs antigen and HBs and HCV antibodies is recommended prior to IFX administration. Because positive conversion of antibodies is possible after IVIG administration, it is desirable for antibody tests to be performed on blood samples before treatment with IVIG.
(3) Exacerbation of Heart Failure
In adults, IFX is contraindicated for cardiac function in New York Heart Association (NYHA) Class III and IV, because worsening of heart failure or death has been reported. In KD patients with heart failure, IFX should be avoided and other treatments should be considered.
(4) Administration to Children under 1 Year of Age, and the Interval between IFX Administration and Live Vaccines
According to the package insert, caution is necessary for the use of IFX in infants under 1 year of age because no domestic clinical trials have been conducted. In addition, the “Guide on Regulatory Approval and Specifications for Infliximab for the Treatment of Acute Kawasaki Disease Refractory to Other Existing Treatment”96) published by the Kawasaki Disease Society of Japan, the Japanese Society of Pediatric Cardiology and Cardiac Surgery, and the Japanese Society of Pediatric Rheumatology recommends that infliximab be withheld for at least 6 months after BCG vaccination and at least 3 months after other live vaccinations.
(5) Others
The development of malignancy and impaired myelination have been reported with long-term IFX treatment in other diseases, but there have been no reports of these side effects in KD patients with a single dose of IFX. Brain magnetic resonance imaging (MRI) for 14 KD patients in the long term, a median 23 months after disease onset, did not show myelination impairment.97)
B. Others
Although there is no insurance coverage and the evidence of its use in KD is insufficient, soluble TNF-α receptor antagonists (etanercept), anti-IL-6 receptor antibody (tocilizumab), and IL-1 receptor antagonist (anakinra) have been reported as biological agents. Etanercept is characterized by its short half-life and low risk of side effects of infection. It is expected to be an effective treatment option in the future because the time of vaccination is at a favorable age of KD onset. In a double-blind, randomized, controlled trial comparing IVIG alone with IVIG plus etanercept subcutaneous injection as the first-line therapy in 2019,98) the change in coronary artery diameter was significantly lower in the IVIG plus etanercept group, although there was no significant difference in the rate of fever resolution. Tocilizumab was used in four KD cases of non-responders to IVIG, and although fever resolution was achieved in all cases, two were reported to develop giant CAA.99) The effectiveness of anakinra in non-responders to IVIG was reported in a case series.100) A Phase I/IIa study of the safety and efficacy of anakinra (ANAKID Trial) is underway in the United States.101)
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Additional treatment with IFX for non-responders to IVIG | IIa | B |
V. Protease Inhibitors (Table 5)
A. Ulinastatin (UTI)
1. Purpose
The principal action of UTI is to reduce damage to vascular endothelial cells by inhibiting the activity of proteolytic enzymes and inflammatory cytokines released from neutrophils.
2. Mechanism of Action
UTI is a human urinary trypsin inhibitor, highly purified from human urine, and a polyvalent enzyme inhibitor (a serine protease inhibitor). It has a molecular weight of 67,000 and blocks various protein-degrading pancreatic enzymes, including trypsin. It is thought to be derived from inter-α-trypsin inhibitors in the blood. UTI is produced by many organs, including the liver, kidney, pancreas, lungs, heart, adrenal glands, stomach, large intestine, brain, and testes.
(1) Suppression of TNF-α
UTI suppresses the production and secretion of inflammatory cytokines, for example, TNF-α, IL-6, and IL-8, from neutrophils or TNF-α from monocytes.102) It also inhibits the expression of ICAM (intercellular adhesion molecule)-1 on the surface of vascular endothelial cells activated by TNF-α, thereby playing a protective role for endothelial cells.
(2) Inhibition of Neutrophil Elastase
Neutrophils release elastase and other proteolytic enzymes. UTI suppresses the secretion of various proteases from neutrophils via stabilization of the lysosome membrane, and inhibits the activity of released neutrophil elastase, resulting in the removal of free radicals (antioxidant effect) and the decrease in the activity of cytokines and adhesion molecules. It also blocks the release of myocardial inhibitory factors containing TNF-α and hypercoagulopathy.103)
3. Indications
The use of UTI to treat KD or other diseases in children is not covered by insurance. Its use may be considered in combination with IVIG as an initial treatment or as an additional treatment for non-responders to IVIG.
4. Dosage and Usage
The optimal dosage for pediatric patients has not been established. Several reports show that a dose of 5,000 U/kg given 3 to 6 times/day, not exceeding 300,000 units/day, is suitable for KD patients. The half-life is 40 min, with an intravenous infusion of 300,000 units/10 mL.
5. Usefulness
In acute KD, UTI inhibits the mRNA transcription of prostaglandin H2 and thromboxane A2 in neutrophils,104) and also prevents neutrophil-induced damage to endothelial cells.105) After the first report of its use in 1993,106) several case series studies reported the following: (i) its effect as a single agent in mild cases, (ii) its reduction effect in combination use with IVIG, and (iii) its effect as additional treatment in non-responders or recurrent cases.107) Although these studies had a small number of patients and there have been no prospective clinical trials, UTI has been historically developed as an additional option for treating non-responders to IVIG.3)
Subsequently, the initial treatment with a combination of UTI and IVIG was reported to reduce the frequency of additional treatment for non-responders to IVIG (adjusted OR 0.30, 95% CI 0.20–0.44) and the incidence of CAA (adjusted OR 0.32, 95% CI 0.17–0.60) in a retrospective study.25) In the subgroup analysis, this effect was higher in high-risk patients with 7 points of Kobayashi score or more (adjusted OR for CAA 0.21, 95% CI 0.08–0.57). As an additional treatment for non-responders to IVIG, a case series study reported that no CAA was observed in 7 patients who received additional IVIG with UTI.108)
6. Side Effects
Patients with i) drug hypersensitivity or a history of drug hypersensitivity, ii) a predisposition to hypersensitivity, and iii) a history of UTI use are listed in the package insert as eligible for cautious administration. The side effects of UTI include anaphylactic shock (incidence unknown), hepatic dysfunction (0.5%), leukopenia (0.2%), hypersensitivity such as rash and pruritus (0.1%), diarrhea (0.1%), angialgia (0.1%), transient elevation of AST and ALT, eosinophilia, and vascular pain at the injection site. Because mixing UTI with IVIG causes turbidity, multiple intravenous routes should be used, or IVIG administration should be stopped temporarily and the IVIG route flushed with saline before and after UTI administration on the same route.
B. Others
Sivelestat sodium hydrate (SSH), a more potent and selective inhibitor of neutrophil elastase, is another protease inhibitor like UTI. SSH is indicated for the treatment of acute lung injury associated with systemic inflammatory response syndromes and has an off-label use for KD. In some reports, SSH was used in combination with IVIG for initial treatment or as an additional treatment for non-responders to IVIG.109, 110) Although the optimal dosage for pediatric patients has not been established, several reports show continuous intravenous infusion of 0.2 mg/kg/h in KD. There is no established evidence regarding the indication, dosage, or duration of administration at this time.
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Initial treatment with IVIG plus UTI | IIb | C |
| Additional treatment with IVIG plus UTI for non-responders to IVIG | IIb | C |
VI. Plasma Exchange (PE) (Table 5)
1. Purpose
PE can correct hypercytokinemia through the direct removal of the inflammatory cytokines and chemokines that are involved in the pathogenesis of KD.
2. Mechanism of Actions
Inflammatory cytokines such as TNF-α and IL-1β play a major role in the inflammation of KD, as shown by the efficacy of biological agents and animal models. PE can remove TNF-α, IL-1β, IL-6, IL-17, and GCS-F, calming down systemic inflammation.111) Additionally, the suppression of activated monocytes and an increase in regulatory T cells have also been reported.112)
3. Indications
PE is principally indicated in IVIG non-responders. PE is an invasive therapy which requirs deep sedation and often ventilator or intensive care unit management. Therefore, it is mainly considered in the following cases: i) patients who are resistant to or cannot receive the standard treatment for IVIG non-responders, including infliximab or steroids, ii) those who have complications including severe infections or KDSS.
4. Dosage and Usage
Using 5% of albumin or fresh-frozen plasma (FFP) for replacement fluid, the amount of replacement in each treatment is approximately 1 to 1.5 times the total circulating plasma volume. Total circulating plasma volume (mL) is calculated by the following formula; [body weight (kg)/13×(1- hematocrit (%)/100)×1,000]. A double-lumen catheter is placed in the external jugular vein, femoral vein, or subclavian vein. Treatment time is approximately 2 h. For patients with KDSS, the time of the initial treatment should be extended to prevent sudden changes in circulation status. As anticoagulation therapy, administer one shot of sodium heparin (15–30 U/kg) intravenously at the start of PE, followed by 10–30 U/kg/h, maintaining an ACT (activated clotting time) of around 200 s. If hypotension is a concern, especially in children less than 10 kg, start PE after filling the circuit with a mixture of packed red blood cells and 5% albumin in equal proportions.113) When FFP is mainly used for replacement, it can cause hypocalcemia (numbness of the lips and fingers, nausea, vomiting, convulsions, and unconsciousness) because it contains sodium citrate. Ionized Ca should be monitored periodically during PE, and should be corrected with gluconate Ca if necessary. PE can be performed up to 6 consecutive days until fever resolution.
5. Usefulness
PE has a long history, dating back to the pre-IVIG era.114) According to the Nationwide Survey,1) 60 to 80 KD patients receive PE every year, even as IFX has been actively introduced. PE is often attempted as the last resort when other treatment options fail. As PE is generally limited to severe patients, there have been no prospective clinical trials to validate its efficacy. Several retrospective cohort studies are all that have been reported.115, 116)
One study examined 125 non-responders to IVIG treated with PE. Of the 105 patients without CAA at the start of PE, 21 patients developed CAA during the acute phase, but none had CAA 1 year later. Of the 14 patients who already had dilatation at the start of PE, 3 patients developed aneurysm (1 with giant aneurysm) and 11 patients developed dilatation during the acute phase; however, only 2 patients had aneurysms (1 with giant aneurysm) 1 year later. In contrast, in the 6 patients who had already formed aneurysms at the start of PE, CAA had disappeared in 2 patients but giant aneurysms remained in 4 patients 1 year later. The median number of days of PE was 3 days (range 1–6 days).116) PE requires several days until fever resolution. Because there is a risk that CAA will appear or progress during PE, it is preferable to start before the appearance of CAA, similar to other treatments. The efficacy of PE has also been described for IVIG-refractory KDSS, for which no treatment has ever been established.117)
6. Side Effects
Possible side effects of PE are hypotension/shock, hemorrhage, anemia, and hypothermia associated with extracorporeal circulation, coagulation disorders associated with albumin replacement, allergic reactions, hypocalcemia, and infection caused by FFP, thrombosis, or vascular injury in catheterized vessels, and complications associated with deep sedation.
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Additional treatment with PE for non-responders to IVIG | IIa | C |
VII. Antiplatelets (Table 6)
Table 6 Antiplatelet, anticoagulant, thrombolytic agents, antianginal agents, and others| General name | Mechanisms | Treatment route, dosage, and usage | Main side effects | Precautions |
|---|
| Aspirin | Cyclooxygenase inhibition. | Oral dose of 30 to 50 mg/kg/day, in 3 divided doses, and the dose should be reduced to 3 to 5 mg/kg once daily after defervescence without recurrent fever for 48 to 72 h. | Liver dysfunction, intracranial/gastrointestinal/nasal bleeding, shock, anaphylaxis, toxic epidermal necrolysis/cutaneous mucous membrane eye syndrome, gastrointestinal ulcer/symptoms, etc. | |
| Dipyridamole | Platelet aggregation inhibition by inhibition of phosphodiesterase. | 2–5 mg/kg/day, in 3 divided doses. | Headache (0.9–4.4%), palpitations (0.4–0.6%), and severe side effects including worsening of angina symptoms (<0.1%), tendency to bleed (incidence unknown), etc. | No insurance coverage for children, but reimbursement is available. |
| Ticlopidine | Platelet aggregation inhibition by increased platelet adenylate cyclase activity via ADP receptor inhibition. | 2–5 mg/kg/day, in 3 divided doses. | TTP, agranulocytosis, severe liver damage (incidence unknown), etc. | Indications for treatment should be carefully examined. Blood tests are required every 2 weeks during initial treatment. No insurance coverage for children. |
| Clopidogrel | Platelet aggregation inhibition by increased platelet adenylate cyclase activity via ADP receptor inhibition. | 0.2–1 mg/kg/day, once daily | Bleeding (intracranial bleeding ≤1%, gastrointestinal bleeding ≤1%, and incidence of others unknown), gastroduodenal ulcers, thrombotic thrombocytopenia, interstitial pneumonia, pancytopenia, toxic epidermal necrolysis/cutaneous mucous membrane eye syndrome, rhabdomyolysis, etc. | Fewer side effects of liver dysfunction and agranulocytosis compared to ticlopidine. No insurance coverage for children. |
| Unfractionated heparin | Displays anticoagulant effect by activating AT-III, a factor in physiological inhibition of coagulation factors II, VII, IX, X, XI, and XII. | Continuous intravenous infusion with 10–20 units/kg/h with or without antecedent single dose of 50 units/kg. | Hemorrhage as the principal side effect (incidence unknown), HIT (incidence unknown), liver dysfunction (0.1 to <5%), rash (incidence unknown), hair loss/vitiligo (incidence unknown), etc. | APTT should be controlled within 46–70 s (1.5–2.5 times as controls). No insurance coverage for children. |
| Low-molecular-weight heparin (Dalteparin) | Displays anticoagulant effect mainly by inhibiting factor Xa through AT-III. | Continuous intravenous infusion with 75 units/kg/day over 24 h for DIC, and intravenous dose of 15–20 units/kg at the start, followed by 7.5–10 units/kg/h of continuous intarvenously or subcutaneously for hemodialysis. | Lower incidence of hemorrhage than unfractionated heparin. Subcutaneous bleeding (3.8%), HIT (0.4%), headache/vertigo (1–<10%), constipation/diarrhea (1–<10%), liver dysfunction (1–<10%), etc. | Same as above. No insurance coverage for children. |
| Warfarin | Achieves anticoagulant effect by inhibiting biosynthesis of vitamin K-dependent coagulation factors II, VII, IX, and X. | 0.16 mg/kg/day for <12 mo of age, and 0.04–0.10 mg/kg day for ≥1 yr and <15 yr of age in a single dose. | Hemorrhage (incidence unknown), allergic reactions (incidence unknown), liver dysfunction/jaundice (incidence unknown), etc. | PT-INR should be adjusted to 2.0–2.5 and thrombotest to 10–25%. Because warfarin passes through the placenta and is teratogenic, it is contraindicated for pregnant women in their first trimester. Covered by insurance for children. |
| Urokinase | Degrades fibrin by activation of plasmin. | Systemic treatment 10,000–16,000 units/kg (max 960,000 units), intravenously infused over 30–60 min. Intracoronary thrombolysis 4,000 units/kg over 10 min, max 4 times. | Hemorrhagic cerebral infarction (0.1 to <0.5%), cerebral hemorrhage (<0.1%), gastrointestinal hemorrhage (<0.1%), liver dysfunction (<0.1%), rash and other allergic reactions (<0.1%) etc. | Additive effect with heparin, warfarin, aspirin, dipyridamole, ticlopidine, and other t-PA medications, leading to possible increased risk of hemorrhage. When given with aprotinin medications, the capacity for fibrinolysis may be weakened. No insurance coverage for children. |
| Alteplase | Same as above. | 290,000–435,000 units/kg (0.5–0.75 mg/kg); first administer 10% of the total volume of medication intravenously for 1–2 min, and the remaining volume by drip infusion over 60 min. | Tendency to bleed, including cerebral hemorrhage (0.4%), gastrointestinal hemorrhage (0.6%), pulmonary hemorrhage (0.08%), severe arrhythmias such as ventricular fibrillation (0.08%), shock/anaphylactic symptoms (0.1%), liver dysfunction (0.1 to <1.0%) etc. | Increased risk of hemorrhage when given with other thrombolytics, anticoagulants, antiplatelet medications etc. No insurance coverage for children. |
| Monteplase | Its half-life, affinity for fibrin, and plasminogen activator activity are greater than those of alteplase. | 27,500 units/kg, given intravenously over 2–3 min. | Tendency to bleed including cerebral/gastrointestinal hemorrhage (0.1–<5%) and pulmonary hemorrhage (incidence unknown), cardiac rupture/perforation of intraventricular septum (0.1–<5%), arrhythmias such as premature ventricular contraction, ventricular tachycardia, and ventricular fibrillation (0.1–<0.5%) after reperfusion, shock/anaphylactic symptoms (incidence unknown), liver dysfunction (0.1–<0.5%), etc. | Same as above. No insurance coverage for children. |
| Propranorol | β-receptor (β1, 2 non-selective) competitive blockade. | Start at a low dose of 0.5–2.0 mg/kg/day, divided into 3–4 doses. If the effect is inadequate, the dose can be increased to 4.0 mg/kg/day (max 90 mg/day). | Congestive heart failure (or worse), bradycardia, peripheral ischemia (e.g., Raynaud's-like symptoms), atrioventricular block (<0.1–5%), orthostatic hypotension with fainting (<0.1%), agranulocytosis, thrombocytopenia, purpura (<0.1%), bronchospasm (<0.1–5%), dyspnea, wheezing (<0.1%), etc. | Rapid onset with short duration of action. Strong inhibitory effect on renin secretion. Covered by insurance for children with coronary artery stenosis associated with myocardial ischemia, post-myocardial infarction, and heart failure, but not angina. |
| Carvedilol | Same as above. | Start with 0.05 mg/kg/dose, twice a day, and slowly increase to 0.1–0.5 mg/kg/dose, twice a day. Do not exceed the maximum dose for adults (20 mg). | Vertigo (8.9%), worsening of heart failure (9.4%), palpitations (2.7%), bradycardia (2.7%), hypotension (1.9%), worsening of diabetes mellitus (2.3%), generalized lethargy (1.9%), etc. | α1 receptor blocking effect and mild β blocking effect. Reimbursement is available for children with chronic heart failure. |
| Metoprolol | β-receptor (β1 selective) competitive blockade. | 0.5–1.0 mg/kg/dose, twice a day. | Cardiogenic shock, congestive heart failure, atrioventricular block, bradycardia, sinus dysfunction, liver dysfunction, etc (incidence unknown). | No insurance coverage for children. |
| ADP, adenosine diphosphate; APTT, activated partial thromboplastin time; AT-III, anti-thrombin III; DIC, disseminated intravascular coagulation; HIT, heparin-induced thrombocytopenia; IVIG, intravenous immunoglobulin; PT-INR, prothrombin time international normalized ratio; t-PA, tissue plasminogen activator; TTP, thrombotic thrombocytopenic purpura |
A. Aspirin (ASA)
1. Purpose
Use a moderate dose of ASA for its anti-inflammatory effect until several days after the fever resolves. Next, reduce to a low dose for its antiplatelet effect, and continue use for 2 to 3 months after the onset of KD. ASA should be continued in patients with CAA, as it plays an important role in antithrombotic therapy in the remote period.
2. Mechanism of Action
ASA inhibits cyclooxygenase and suppresses thromboxane A2 (TXA2) and prostaglandin E2 (PGE2). ASA acetylates cyclooxygenase, which prevents it from binding to its original substrate (arachidonic acid), thus suppressing the production of TXA2. Even at low doses, ASA suppresses the strong platelet aggregation effect of TXA2. Low-dose ASA does not suppress PGE2 production.
3. Indications
All dosage forms of ASA are covered by insurance for KD, including cardiovascular sequelae caused by KD.
4. Dosage and Usage
In Japan, a moderate dose of ASA (30–50 mg/kg/day, in divided doses 3 times a day) is recommended in the acute phase, and this dosage and administration is covered by insurance. A low dose is sufficient to achieve the antiplatelet effect, but a moderate dose is required for the anti-inflammatory effect. The high dose of 80 to 100 mg/kg/day recommended in the United States is not recommended in Japan because Japanese people develop liver dysfunction more frequently. It has been reported that the absorption of ASA is poor in the acute phase and that the blood concentration is insufficient for anti-inflammatory activity, but if ASA is not used, increased platelet aggregation is seen 2 to 4 disease weeks later. Platelet aggregation is suppressed with the administration of 30 to 50 mg/kg/day.118) Usually, if 48 to 72 h have passed since the fever resolves and normal body temperature is maintained, then the dose should be reduced to a low dose (3–5 mg/kg/day, once daily). After dose reduction, use as an antiplatelet drug for 2 to 3 months.
5. Usefulness
Mortality has improved since ASA became widely used for KD in the 1970s.119) A prospective study in the early 1980s120) established the superiority of ASA for improving CAA from the acute to remote stages. Flurbiprofen, an anti-inflammatory drug with the same COX-1 inhibitory effect, was less effective in that study. Since the late 1980s when IVIG therapy was established, ASA has always been used in clinical trials.40–42) Although ASA alone has not been proven to suppress CAA in the acute phase, it has been shown to be useful for suppressing and improving CAA in the remote phase when used in combination with IVIG.
In recent years, the CAA inhibitory effect in the acute phase has been shown to be highly specific to IVIG,121) and there are some reports that low-dose ASA may be used from the early stage.122–125) According to two recent meta-analyses, fever duration (mean difference 0.30, 95% CI 0.02–0.58)126) and incidence of additional treatment (combined risk ratio 1.39, 95% CI 1.00–1.93)127) tend to increase with low doses compared with medium and high doses, but the incidence of CAA does not increase (risk ratio 1.15, 95% CI 0.93–1.43126); risk ratio 0.85, 95% CI 0.63–1.14).127) The results of future prospective studies may lead to reconsideration of the initial treatment dose.
6. Side Effects
If liver enzymes are elevated before treatment, the cause is bile stasis due to biliary edema in KD and there is no problem if ASA is used. If an increase in liver enzymes is observed during the recovery period or subacute period, appropriate measures should be taken, such as dose reduction and discontinuation of ASA.
Other side effects include bleeding and peptic ulcer, which are observed as frequent epistaxis and black stools. Although the frequency is unknown, these side effects may occur in children, requiring dose reduction or discontinuation of ASA. Although the incidence of Reye’s syndrome has decreased, caution is still needed in patients with varicella or influenza. If KD develops in a patient with influenza or varicella, or during the convalescent period, treat with IVIG alone or with anti-inflammatory or antiplatelet drugs other than ASA. Although low-dose ASA is not associated with onset of Reye’s syndrome,11) we recommend prophylactic vaccination against influenza for children taking low-dose ASA.
B. Others
Antiplatelet drugs with different mechanisms of action include dipyridamole (inhibition of phosphodiesterase → increased cAMP concentration in platelets), ticlopidine, and clopidogrel (both: inhibition of ADP receptors → increased platelet adenylate cyclase activity → increased cAMP concentration in platelets). There is little evidence for the use of these drugs for KD treatment, and they are not covered by insurance (in the case of dipyridamole, insurance is reimbursed). There are no reports with high levels of evidence on whether to use another antiplatelet drug in addition to ASA or anticoagulant therapy in patients with CAA, and there are no clear criteria or risk-benefit profiles available.
Refer to Table 6 for doses and instructions for use. Dipyridamole has been used for a long time and headache is a frequent side effect. Ticlopidine should be used with caution because it may have side effects such as thrombotic thrombocytopenic purpura, agranulocytosis, and severe liver damage within 2 months of administration. Evidence has accumulated for the use of clopidogrel in combination with ASA to prevent thrombosis in adult coronary artery disease, and there are reports of dose setting in children.128) The mechanism of action is similar to that of ticlopidine but there is less liver damage. In adults, the combined use of clopidogrel with ASA was effective in preventing myocardial infarction, but cardiovascular mortality did not improve because of increased bleeding events.129)
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Initial treatment with IVIG plus ASA | I | A |
| ASA for CAA | I | C |
| Antiplatelet drugs other than ASA for CAA | IIb | C |
VIII. Other Cardiovascular Drugs (Table 6)
A. Anticoagulants
In acute KD, platelet hyperplasia and activation of the coagulation fibrinolytic system are observed. As a rule, patients without CAA do not need anticoagulants. Especially in cases of giant aneurysms (Z-score ≥10 or aneurysm diameter ≥8 mm), however, anticoagulants are recommended in addition to antiplatelet drugs.130–132) Most infarcts have been reported to occur within 2 years of the onset of KD,133) and anticoagulant treatment during this period should thus be performed with caution. The most used anticoagulant in KD is warfarin, and heparin is used when warfarin is not available. Newer anticoagulants such as direct oral coagulants (DOACs) that inhibit clotting factors independently of vitamin K have emerged and are used in adults with atrial fibrillation, but no evidence has been reported on them and they are used off-label in KD.
A-1. Warfarin
1. Purpose
Prevention of thrombus formation in CAA.
2. Mechanism of Action
Warfarin exerts an anticoagulant effect by inhibiting the synthesis of factors II, VII, IX, and X, which are vitamin K–dependent coagulation factors.
3. Indications
Giant CAA, previous myocardial infarction, and thrombus formation in aneurysm are indications. For thromboembolism, such as myocardial infarction and pulmonary vein thrombosis, children are covered by insurance.
4. Dosage and Usage
Administer 0.16 mg/kg/day for children younger than 12 months, and 0.04 to 0.10 mg/kg/day for children older than 1 year and younger than 15 years, once daily. The effects of warfarin vary greatly among individuals, and genetic polymorphisms in CYP2C9 and VKORC1 have been reported to have a significant effect on warfarin dosage.134) Periodically adjust the dose using the international standardization ratio of prothrombin time (PT-INR) because the effect is variable in the same individual. The Japanese Society of Cardiology Guidelines135) suggest a target level of PT-INR as 2.0 to 2.5.
Warfarin is difficult to control during the acute inflammatory response, and its effect takes several days to develop and stabilize. If CAA is identified in the acute phase and requires immediate anticoagulation, it has been reported that continuous intravenous heparin followed by warfarin may be safer.136)
The effect of warfarin is easily influenced by diet, and is attenuated by vitamin K–rich fermented soybeans, green juice, chlorella, and vitamin K–enriched milk, and enhanced by breastfeeding and poor diet. Drugs that potentiate action include ST mixture, acetaminophen, antimicrobials (e.g., erythromycin), antifungals (e.g., fluconazole), anabolic steroids, amiodarone, and statins. Drugs that attenuate the effects include phenobarbital, carbamazepine, and rifampicin. We recommend that readers refer to Warfarin Appropriate Use Information.137)
5. Usefulness
No large clinical trials have been reported on the efficacy of warfarin in the acute phase of KD. In giant CAA, the combination of ASA and warfarin has been reported to be effective in avoiding cardiac events in the distant phase, although these reports were retrospective studies.130–132) The use of warfarin in small aneurysms is not recommended, but its use in medium aneurysms may be considered depending on the status of the aneurysm.12, 135) If giant CAA regresses to a moderate aneurysm or less, the discontinuation of warfarin may be considered.12, 135) Because of the limitations of warfarin in preventing myocardial infarction,133, 138) however, and because of the problems of bleeding and thrombosis,139) treatment should be decided regarding long-term indications based on risk-benefit considerations.
6. Side Effects
The biggest side effect is bleeding. Nasal and gingival hemorrhages are common, and hypermenorrhea can be present in women. Careful attention must be paid to possible intracranial and intraperitoneal hemorrhage. Warfarin is also known to be teratogenic, causing abnormalities in bone formation, chondrogenesis, and microencephaly when taken during the first 6 to 9 weeks of pregnancy.140) The frequency of occurrence is approximately 5%, however, and the risk is reported to be low at doses of 5 mg or less per day.141)
A-2. Heparin
1. Purpose
Thromboprophylaxis in CAA: if there is a risk for a complication of CAA and early thrombus formation, start with a continuous intravenous heparin infusion that is switched to oral warfarin after the inflammation subsides. Some reports have suggested that it was safer to use heparin than to start with warfarin.136)
2. Mechanism of Action
Unfractionated heparin was extracted from the small intestinal mucosa, liver, and lungs of healthy food-producing animals. Binding to thrombin (IIa) and antithrombin III (ATIII) activates ATIII, and blocks factors IIa, IXa–XIIa, and kallikrein, thereby inhibiting blood clotting. Low-molecular-weight heparin, like unfractionated heparin, binds to ATIII but cannot bind to IIa and exerts its anticoagulant effect by inhibiting factor Xa through ATIII.
3. Indications
The indications include giant CAA, myocardial infarction, and thrombus formation in CAA. The package insert states that the safety and efficacy of the drug in children have not been established.
4. Dosage and Usage
Unfractionated heparin: 10 to 20 units/kg/h by continuous infusion (a single dose of 50 units/kg may be given initially).142) It is recommended that activated partial thromboplastin time (APPT) or activated coagulation time (ACT) be 1.5 to 2.5 times higher than the control value.
Low-molecular-weight heparin: No pediatric dosage has been established. The adult dosage of dalteparin is 75 units/kg/day for continuous intravenous infusion over 24 h for disseminated intravascular coagulopathy (DIC) and 15 to 20 units/kg at the beginning of hemodialysis followed by 7.5 to 10 units/kg/h for continuous intravenous infusion during hemodialysis.
5. Usefulness
Patients with CAA of moderate or giant size who were initially treated with warfarin were reported to have more common severe bleeding compared with those who were treated with heparin at the start followed by switching to warfarin.136)
6. Side Effects
Clinicians should monitor for heparin-induced thrombocytopenia (HIT), bleeding, liver dysfunction, alopecia, rash, and diarrhea. Low-molecular-weight heparin is associated with less HIT and bleeding than unfractionated heparin.
A-3. Direct Oral Anticoagulant (DOAC)
1. Purpose
Prevention of thrombus formation in atrial fibrillation and venous thrombosis.
2. Mechanism of Action
Independent of vitamin K, DOAC exerts an anticoagulant effect by directly inhibiting thrombin and factor Xa. There are two types of drugs: dabigatran (a thrombin inhibitor) and factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban.
3. Indications
In adults, it is indicated for the prevention of ischemic stroke and systemic embolism in patients with non-valvular atrial fibrillation, and for the treatment and prevention of recurrent deep vein thrombosis and pulmonary embolism. Currently, there is no insurance coverage for KD. In the future, it may be an alternative to warfarin and heparin.
4. Dosage and Usage
The adult dose is 150 mg of dabigatran twice daily, 15 mg of rivaroxaban once daily, 5 mg of apixaban twice daily, and 60 mg of edoxaban (30 mg if the patient weighs less than 60 kg) once daily. The dosage for children has not been established.
5. Usefulness
In non-valvular atrial fibrillation and venous embolism, DOAC is generally superior to warfarin in terms of efficacy and safety.143, 144) DOAC has been reported to be inferior to warfarin for both thrombosis and bleeding after valve replacement surgery.145) Unlike warfarin, the advantage is that DOAC is less affected by diet and does not require monitoring, but the disadvantages are that monitoring its effect is difficult, its half-life is shorter than that of warfarin, its effect is weakened if a dose is forgotten, and there is no neutralizing agent for emergencies.146)
6. Side Effects
Bleeding (gastrointestinal and intracranial hemorrhage), interstitial pneumonia, anaphylaxis, acute liver failure, etc.
B. Thrombolytics
1. Purpose
Myocardial infarction after the onset of KD often occurs within 2 years,133) mostly due to acute coronary artery occlusion caused by thrombus in the aneurysm. Reperfusion therapy should be administered promptly within 12 h of the onset of myocardial infarction in order to reduce the extent of cardiac dysfunction and decrease the risk of cardiac deterioration.147) Although thrombus aspiration by percutaneous coronary intervention (PCI) is performed in adults, thrombolytic therapy is often indicated in children due to their smaller size and fewer bleeding complications148) and is recommended by the guidelines of the Japanese Society of Cardiology.135) In adults, thrombolysis is recommended if primary PCI cannot be performed within 12 h of onset and within 2 h of initial contact with the patient.147) Thrombolysis is also indicated when asymptomatic thrombi are identified in CAA and myocardial infarction is threatened.
2. Mechanism of Action
Thrombolytic drugs are protein preparations belonging to the plasminogen activator (PA), an enzyme that activates the fibrinolytic system. Activation of the fibrinolytic system is initiated by turning plasminogen into plasmin, which dissolves the thrombus by degrading fibrin, a component of the thrombus. Thrombolytic agents include first-generation urokinase and recombinantly synthesized tissue-type plasminogen activator (tPA) whereas tPA includes second-generation alteplase and third-generation modified tPA monteplase. Urokinase has low fibrin affinity and elevates fibrinolytic activity whereas alteplase is a natural type of tPA with high fibrin affinity and it binds to the fibrin molecule, activating plasmin on the fibrin thrombus, degrading the fibrin molecule, and dissolving the thrombus. Monteplase replaces the amino acid sequence of tPA to increase the blood half-life, allowing for a reduction in total dose and rapid single dose administration.
3. Indications
Acute myocardial infarction and thrombosis in CAA. Children are not covered by insurance.
4. Dosage and Usage
The efficacy of intravenous and intracoronary administration of thrombolysis is comparable. Only urokinase is indicated for intracoronary administration.
- (1) Urokinase
- Intravenous: 10,000 to 16,000 units/kg (up to 960,000 units) administered intravenously over 30 to 60 min.
- Intracoronary: 0.4 million units/kg infused over 10 mins. Up to 4 times.
- (2) Alteplase
- Intravenous administration: 290,000 to 435,000 units/kg (0.5–0.75 mg/kg); 10% of the total dose administered intravenously over 1 to 2 min and the remainder intravenously in 1 h.
- (3) Monteplase
- Intravenous: 27,500 units/kg administered intravenously over 2 to 3 min.
5. Usefulness
In a case series of 50 patients with acute coronary syndromes in adults with pre-existing KD, intracoronary thrombolysis was performed in 11 patients and reported to be effective in 9 patients.149) According to a nationwide survey by Harada et al.,148) intravenous thrombolytic therapy was effective in 14 out of 20 patients with aneurysm diameter of 10 mm or less, and intracoronary administration was effective in patients with aneurysm diameter of more than 10 mm.
6. Side Effects
Cerebral hemorrhage, hemorrhagic infarction, gastrointestinal hemorrhage, pulmonary hemorrhage, anaphylaxis, shock, etc.
C. Anti-Angina Drugs and Coronary Dilators
Angina symptoms in the acute phase are rare. Early thrombotic myocardial infarction and coronary artery rupture within 1 month of onset have been reported, however. For the treatment of angina pectoris, it is important to reduce the cardiac load (slowing the heart rate, reducing preload and afterload, and increasing coronary blood flow), and this can be achieved with the use of β-blockers, Ca antagonists, and nitrates. If coronary artery rupture is thought to be imminent in the acute phase, adequate sedation and antihypertensive measures, in addition to KD treatment, are necessary, and β-blockers and Ca antagonists should be considered.
C-1. β-blockers
β-blockers are the first-line choice for an anti-angina drug that competitively inhibits β-receptors and suppresses their effects, such as contractile enhancement and smooth muscle relaxation. If there is coronary spasm, care must be taken to ensure that the α-receptor effect is predominant, and coronary tonus may be increased, exacerbating coronary angina pectoris. Because glycogen is degraded in the liver via the β2-receptor, and β-blockers inhibit this process, attention should be paid to the development of hypoglycemia, especially in newborns and infants.
Propranolol is the only drug that is covered by insurance for the treatment of coronary artery stenosis with myocardial ischemia, post-myocardial infarction, heart failure, and arrhythmia (but not angina) in children. Carvedilol use is off-label for children, but is reimbursable. Because of the potential to cause heart failure, start small doses and set maintenance doses for individual patients based on tolerability and therapeutic benefit.
- (1) Propranolol
- Start at a low dose of 0.5 to 2.0 mg/kg/day, divided into 3 to 4 doses per day. If the effect is inadequate, the dose can be increased to 4.0 mg/kg/day, but is not to exceed 90 mg/day.
- (2) Carvedilol
- Start at 0.05 mg/kg twice daily, and gradually increase to 0.1 to 0.4 mg/kg twice daily (do not exceed the maximum adult dose of 20 mg/day), administered orally.
- (3) Metoprolol
- A dose of 1 to 2 mg/kg/day, divided into 2 to 3 doses per day, administered orally.
C-2. Calcium Antagonists
Ca antagonists inhibit Ca2+ influx in vascular smooth muscle cells and are effective in preventing coronary spasm, making them the first-line treatment for coronary angina.147) Myocardial infarctions in KD occur at rest and during sleep, and may be associated with coronary spasm.150) If coronary artery rupture is thought to be imminent in the acute phase, adequate sedation and antihypertensive measures, in addition to KD treatment, are necessary, and β-blockers and Ca antagonists should be considered. Typical drugs include amlodipine (0.06–0.3 mg/kg/day orally once a day for hypertension in patients aged 6 years and older), nifedipine (1–2 mg/kg/day orally in divided doses 3 times a day, or once or twice a day for extended release drugs), and diltiazem (1.5 mg/kg/day orally in divided doses 3 times a day). Among them, only amlodipine is covered by insurance in children aged 6 years and older for the treatment of hypertension, and nifedipine and diltiazem are off-label for children. Myocardial contraction in immature myocardium is highly dependent on extracellular Ca, and diltiazem, in particular, is contraindicated in patients up to early infancy because it also has a blocking effect on cardiomyocyte L-type Ca2+ channels.
C-3. Nitrates
Nitrates increase coronary artery blood flow due to coronary artery dilation and preload reduction, and decrease left ventricular workload due to pre- and after-load reduction, and thereby improve myocardial ischemia. Sublingual and oral administration of nitrates is tried at the onset of angina pectoris and myocardial infarction.151) Avoid aimless use, as tolerance develops with long-term use. Sublingual nitroglycerin tablets (1–2 tablets, 0.3–0.6 mg, for adults and 1/2 to 1/3 of a tablet for children, depending on body size) and continuous intravenous infusion (0.1–20 µg/kg/min) are covered by insurance for children.
Classification and evidence levels| Treatments | Classification | Evidence levels |
|---|
| Warfarin in combination with ASA for giant CAA | I | C |
| Continuous intravenous heparin therapy for giant CAA | IIa | C |
| Continuous intravenous heparin therapy with ASA for coronary artery embolism | I | C |
| Thrombolytic therapy for thrombotic myocardial infarction in children | I | C |