Beta-blockers are widely used in adults and children for treating cardiovascular disease such as arrhythmia and heart failure. The effectiveness of beta-blockers has recently led to an increase in their use in children.1–3) In pediatric medicine in particular, beta-blockers have been used to prevent anoxia in patients with tetralogy of Fallot, and their effectiveness in treating this condition is widely recognized.
Well-known side effects of beta-blockers include hypotension and the exacerbation of bradycardia and heart failure, and while rare, incidences of hypoglycemia have also been reported.4–12) Hypoglycemia can cause severe sequelae, and while the use of insulin is considered a risk factor in adult diabetic patients,13–15) risk factors in children are unclear.
In the present study, we included pediatric patients with tetralogy of Fallot as a typical disease for which beta-blockers are used and retrospectively examined the incidence of hypoglycemia and risk factors.
In patients using beta-blockers who were examined and diagnosed with tetralogy of Fallot at our department between April 1983 and January 2011, we retrospectively examined the presence or absence of hypoglycemia on the basis of medical records.
The sample included 422 patients with tetralogy of Fallot. They were divided into three groups on the basis of hemodynamics and whether beta-blockers were used. The three groups were as follows: the pulmonary atresia group not using beta-blockers (PA group; 116 patients), the group with tetralogy of Fallot using beta-blockers (TOFβ+ group; 214 patients), and the group with tetralogy of Fallot not using beta-blockers (TOFβ− group; 92 patients. The use of beta-blockers was initiated for patients exhibiting anoxia, those who had an extremely narrow right ventricular outflow tract, and those who were thought to have a high risk of anoxia.
Patients with hypoglycemic attacks were defined as those who visited the hospital for the chief complaint of disturbance of consciousness, seizures, and severe fatigue, at which time blood glucose measurement revealed hypoglycemia. Hypoglycemia was determined when the following criteria were met: blood glucose level of ≤40 mg/dL or ≤50 mg/dL and the following Whipple’s trias (clinical symptoms presented, low blood glucose, and blood glucose levels normalized with sugar supplements).16, 17)
All patients who exhibited hypoglycemia were in the TOFβ+ group. Therefore, patients with hypoglycemic attacks were classified in the TOFβ+H group, and those in the TOFβ+ group who did not exhibit hypoglycemia were classified in the TOFβ+E group. To evaluate risk factors for hypoglycemia, we compared the following characteristics: blood glucose levels, symptoms, physical stature, presence or absence of congestive heart failure, degree of oxygen saturation at the time of hypoglycemic attack, type and dose of beta-blocker used, presence or absence of complications, cause of onset, treatment, and presence or absence of sequelae. Heart failure was determined upon the observation of at least two of the following factors: retractive breathing, tachypnea, hepatomegalia, and insufficient intake milk.18)
Results are presented as mean±standard deviation (SD) and median for the start and completion times of beta-blocker use (minimum–maximum). To compare patients with hypoglycemic attacks with those without, data were analyzed after matching for the date of birth and age at onset in patients with hypoglycemia. To evaluate background factors, the difference between TOFβ+E and TOFβ+ H groups was statistically analyzed using Student’s t-test and the chi-square test. To evaluate the effect of each factor, conditional logistic regression analysis was performed. When comparing the beta-blocker dose used, potencies were converted to carteolol=1 and propranolol/metoprolol=0.2 before the doses used were compared.19)
1. Patients (Tables 1, 2)
Table 1 The distributions of groups and hypoglycemia probands in the TOF 422 cases: the number of patients (percentage). All of the probands were in the β+ group treated with beta-blockers (7.5%)| Group | TOF |
|---|
| β+ | β− | PA | Total |
|---|
| Case | 214 | 92 | 116 | 422 |
| Hypoglycemia probands (%) | 16 (7.5%) | 0 (0.0%) | 0 (0.0%) | 16 (3.8%) |
| β+=patients treated with beta-blocker, β−=patients treated without beta-blocker, PA=patients with pulmonary atresia |
Table 2 Demographic characteristics of cases with hypoglycemia: all subjects took carteolol and became symptomatic early in the morning| Case | Sex | G.A. | Birth weight | Age (Mo) | Bw (kg) | Kaup | Min PG | LOC | Seizure | Other symptom | Trigger | Chromosomal analysis |
|---|
| 1 | M | 39 | 2,765 | 7 | 8.1 | 18.0 | 19 | + | + | Hypothermia | Unknown | |
| 2 | M | 39 | 2,520 | 14 | 7.3 | 14.7 | 3 | + | + | | Ordered fasting | |
| 3 | F | 41 | 2,700 | 43 | 12.0 | 13.8 | 23 | + | + | | AGE | |
| 4 | M | 38 | 3,152 | 14 | 9.3 | 16.8 | 17 | + | + | Hypothermia | Cold | |
| 5 | M | 39 | 3,192 | 27 | 12.6 | 17.0 | 15 | + | + | | AGE | |
| 6 | M | 40 | 2,696 | 12 | 7.2 | 15.3 | 38 | + | + | Sweating | Cold | |
| 7 | F | 41 | 3,230 | 22 | 10.9 | 17.0 | 13 | + | + | Hypothermia | Cold | |
| 8 | F | 39 | 3,046 | 29 | 10.7 | 15.9 | 33 | + | + | Sweating | Fast | 22q11.2 deletion |
| 9 | F | 34 | 1,282 | 33 | 10.1 | 14.2 | 25 | + | + | | Fast | |
| 10 | M | 40 | 2,984 | 25 | 9.5 | 14.8 | 20 | + | + | Hypothermia | AGE | 22q11.2 deletion |
| 11 | F | 39 | 2,735 | 59 | 14.1 | 15.0 | 25 | + | + | | AGE | 22q11.2 deletion |
| 12 | F | 39 | 2,732 | 30 | 11.2 | 15.7 | 35 | + | + | | Ordered fasting | |
| 13 | M | 40 | 3,058 | 32 | 9.8 | 15.3 | 50 | + | + | | AGE | 16q12.1 deletion |
| 14 | M | 40 | 2,636 | 21 | 9.6 | 13.5 | 29 | + | + | Tachypnea | AGE | |
| 15 | F | 38 | 2,932 | 50 | 10.0 | 11.9 | 48 | + | + | | Cold | 22q11.2 deletion |
| 16 | M | 37 | 2,405 | 16 | 7.8 | 14.3 | 19 | + | + | Hypothermia | Unknown | 22q11.2 deletion |
| G.A.=gestational age, Birth weight (gram), Mo=month, Bw=body weight, kg=kilogram, Kaup=Kaup index: Bw (g)×10/Ht (cm)2, Min PG=minimum plasma glucose, LOC=loss of consciousness, TOFβ+=patients with Tetralogy of Fallot treated with beta-blocker, M=male, F=female, AGE=acute gastroenterocolitis |
Among the 422 patients with tetralogy of Fallot, hypoglycemic attacks were observed in 16. All these patients were using beta-blockers; hypoglycemic attacks were observed in 7.5% of the patients in the TOFβ+ group and 3.8% of the patients overall. There were no patients with hypoglycemic attacks in the TOFβ− and PA groups. The mean blood glucose level on the appearance of symptoms was 26.4±14.1 mg/dL.
2. Background Factors at Birth (Gestational Age, Birth Weight, and Chromosomal Abnormalities) (Tables 2, 3)
Table 3 Comparison of characteristics between the groups: 1, 2) β+H was lower the presence of anoxic spell without administration significantly1) and with administration than β+E2). 3) β+H was significantly higher SpO2 than β+E | β+ | p |
|---|
| H | E |
|---|
| Number | 16 | 198 | |
| Sex (Male/Female) | 9/7 | 99/99 | 0.41 |
| Gestational ages (weeks) | 38.9±1.7 | 38.6±1.7 | 0.37 |
| Birth weight (g) | 2,754.1±462.1 | 2,911.7±516.3 | 0.24 |
| Chromosomal analysis (n) | 21tri; 0, 22qdel; 5, other; 1 | 21tri; 24, 22qdel; 8, other; 7 | 0.09 |
| Other features (n) | 2 | 12 | 0.28 |
| Combined medicine (n) | 5 | 41 | 0.25 |
| Starting date of administration (months) | 2.5 (0–42.0) | 4.0 (0–54) | 0.50 |
| End date of administration (months) | 36.0 (12–149.0) | 30.0 (2–324) | 0.45 |
| Month of onset | 27.1±14.2 | 26.8±21.2 | 0.95 |
| Dose (mg/kg/day) | 0.25±0.04 | 0.25±0.06 | 0.94 |
| Presence of anoxic spell without administration (n) | 1 | 54 | 0.051) |
| Presence of anoxic spell with administration (n) | 2 | 57 | 0.132) |
| Kaup index | 15.2±1.5 | 15.7±1.5 | 0.23 |
| BNP (pg/mL) | 18.7 (7.4–56.2) | 19.0 (2.6–88.0) | 0.78 |
| SpO2 (%) | 86.4±7.6 | 81.6±9.0 | 0.043) |
| β+=patients treated with beta-blocker, H=hypoglycemia, E=euglycemia, 21tri=Trisomy 21, 22qdel=22q11.2deletion |
In the TOFβ+H group, the mean gestational age was 38.9±1.7 weeks and the mean birth weight was 2,754.1±462.1 g. Patient 9 was a premature baby delivered at 34 weeks and weighing 1,282 g. Genetic complications included 22q11.2 deletion syndrome in 5 patients and 16q12.1 deletion syndrome in 1 patient. As a concurrent illness, VACTERL association was observed in 1 patient and 1 patient had undergone surgery for pyloric stenosis. Compared to the TOFβ+ group, in the TOFβ+H group, there were no differences in gestational age and birth weight and no difference in the incidence of genetic complications (p=0.09).
3. Age and Gender (Table 3)
In the TOFβ+H group, the mean age at hypoglycemia onset was 2.3±1.2 years and the group consisted of 9 male and 7 female patients. There was no difference between the two groups in terms of age at the start of and completion of beta-blocker use or gender.
4. Physical Stature (Kaup Index) (Table 3)
Physical stature was evaluated based on standardized height and body weight. In the TOFβ+H group, the mean standardized height was −1.57±0.95 SD and the standardized body weight was −1.32±0.96 SD Furthermore, the mean Kaup index (reference value: 16–18) was 15.2±1.5, indicating that the patients had a slightly slender build. In the TOFβ+E group, the patients also had a slightly slender build; however, there was no significant difference from the TOFβ+H group (p=0.23).
5. Drug (Beta-blocker Type, Dosage, Concomitant Drugs) (Table 3)
The beta-blockers used in the TOFβ+ group included carteolol in 208 patients, propranolol in 10 patients, and metoprolol in 1 patient. Among these patients, 5 changed to a different drug, with 4 patients changing from propranolol to carteolol to improve medication compliance and 1 patient changing from carteolol to metoprolol due to asthma symptoms. None of the patients who changed drugs exhibited hypoglycemia, and all patients in the TOFβ+H group used carteolol.
In the TOFβ+H group, the median age at the start of beta-blocker use was 2.5 months (range: 0 months–3.5 years), the mean period of use until the onset of hypoglycemia was 21.6±13.4 months, and the mean dose of carteolol administered was 0.25±0.04 mg/kg, with no significant difference observed compared to the TOFβ+E group.
There was no difference observed in the frequency in the use of concomitant drugs such as diuretics and cardiac stimulants.
6. Comparison of the Incidence of Anoxic Seizures Caused by Beta-blocker Use (Table 3)
The incidence of anoxic seizures caused by beta-blocker use was significantly lower prior to the start of use in the TOFβ+H group than in the TOFβ+E group. However, after the start of use, there was no difference observed (pre start: p<0.05, post start: p=0.13).
7. Relationship with Heart Failure (Table 3)
There were no patients with clear symptoms of heart failure in any of the groups. In brain natriuretic peptide (BNP) sampled as an indicator of heart failure, there was no difference observed between the two groups (p=0.78) and no patients exhibited elevated levels.
8. Relationship with Hypoxemia (Table 3)
In the TOFβ+H group, the mean oxygen saturation was 86.4±7.6%, which was significantly higher than that in the TOFβ+E group (p<0.05).
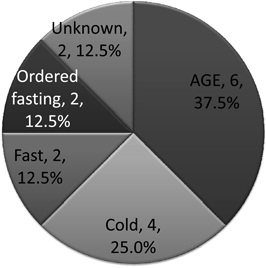
9. Causes (Fig. 1)
In the TOFβ+H group, up to the day prior to onset, 6 patients had gastroenteritis, 4 had common cold symptoms, and 2 complained of loss of appetite and were in a state of poor oral ingestion. On the day of onset, 2 patients had been instructed to fast for performing tests. In 2 patients, no clear cause could be identified. Poor oral ingestion was observed in 14 of 16 patients (87.5%) and was thought to have caused hypoglycemia.
10. Hypoglycemic Symptoms (Table 2)
All patients exhibited early morning loss of consciousness, and 5 patients exhibited seizures, with low body temperature. All symptoms quickly improved with sugar supplementation.
11. Examination of Factors Affecting Low Blood Sugar Levels in Patients Using Beta-blockers (Table 4)
Table 4 Risk factors for hypoglycemia in the TOF 214 cases received beta-blocker determined by conditional logistic analysis. We defined low birth weight as <2.5 kg, “Others” as chromosomal disorders and other features except trisomy 21 and 22q11.2deletion, and a low Kaup index of <16| Variable | Univariable analysis | Multivariable analysis |
|---|
| OR | 95% CI | p | OR | 95% CI | p |
|---|
| Male | 0.54 | 0.17–1.67 | 0.281 | | | |
| Birth weight (g) | 1.00 | 0.99–1.00 | 0.682 | | | |
| Low birth weight (g) | 1.44 | 0.44–4.76 | 0.548 | 1.11 | 0.30–4.14 | 0.869 |
| Trisomy 21 | 1.65 | 0.34–8.02 | 0.533 | 1.99 | 0.38–9.96 | 0.403 |
| 22q11.2 deletion | 3.96 | 0.70–22.46 | 0.120 | 4.41 | 0.68–28.49 | 0.119 |
| Others | 1.42 | 0.34–5.89 | 0.631 | | | |
| Starting date of administraion (month) | 1.00 | 0.94–1.05 | 0.877 | | | |
| The term of administration (months) | 1.03 | 0.96–1.11 | 0.377 | | | |
| Dose (mg/kg/day) | 0.13 | 0.00–4261 | 0.699 | | | |
| The accumulated dosage (mg/kg) | 1.06 | 0.83–1.36 | 0.651 | | | |
| Anoxic spell without administration | 0.21 | 0.02–1.77 | 0.150 | | | |
| Kaup index | 0.81 | 0.55–1.20 | 0.299 | | | |
| Low Kaup index | 1.88 | 0.56–6.32 | 0.309 | 1.90 | 0.54–6.68 | 0.316 |
| BNP (pg/mL) | 0.99 | 0.96–1.03 | 0.671 | | | |
| SpO2 (%) | 1.04 | 0.97–1.12 | 0.241 | | | |
| OR=odds ratio, 95% CI=95% confidence interval |
Only patients using beta-blockers developed hypoglycemia. Therefore, the effect of background factors other than the use of beta-blockers on hypoglycemia was examined by performing conditional logistic regression analysis in the TOFβ+ group. Known exacerbation factors for hypoglycemia were examined; however, we found that none showed a significant difference.
12. Sequelae (Table 2)
Neurological sequelae were observed in 3 patients (18.8% of patients with hypoglycemia and 1.4% of patients in the TOFβ+ group), all of whom were diagnosed as having symptomatic epilepsy and were started on anticonvulsants. One patient presented with severe encephalopathy, and it was not noted at the time of the initial seizure that hypoglycemia was the underlying cause. This patient was examined in 1990, when the side effects of beta-blockers were yet to be determined and proactive preventive measures for hypoglycemia were not adopted. Surgery was deemed inappropriate to treat severe encephalopathy, and the use of beta-blockers was continued, during which time hypoglycemia occurred a second time. The patient did not respond to treatment and died due to cerebral herniation.
13. Outcomes
Excluding 2 patients with severe neurological sequelae, intracardiac repair was performed at a mean age of 3.3±1.4 years and the use of beta-blockers was discontinued. Thereafter, progress was good, with no hypoglycemic symptoms observed.
In the present study, we examined the incidence of hypoglycemia and the use of beta-blockers in infants with tetralogy of Fallot. We observed hypoglycemia in 16 patients, accounting for 7.5% of the patients in the group using beta-blockers. Hypoglycemia has been reported as an adverse event associated with beta-blocker use in only a few cases, and package insert notes have shown that its incidence is less than 0.1% or unknown. The results of the present study showed that the incidence of hypoglycemia was higher than the incidence of ketotic hypoglycemia commonly seen during infancy (incidence of 3.9 per 100,000 individuals)20) and the general incidence of hypoglycemia caused by beta-blockers (noted above).
1. Risk Factors for Hypoglycemia in Patients with Tetralogy of Fallot
It has been reported that in infants, risk factors for low blood glucose include premature birth,21) slim build,22) and poor oral ingestion22) as well as organic diseases, such as diabetes in patients using insulin and sulfonylurea preparations,13–15) cyanotic heart disease,23, 24) and severe heart failure.23, 25)
In the present study, hypoglycemia was not observed in the TOFβ− and PA groups, in which the patients were not using beta-blockers, but was only observed in the TOFβ+ group. The conditional logistic regression analysis conducted in the TOFβ+ group revealed no significant difference in known exacerbation factors for low blood glucose, and no impact was observed in terms of beta-blocker dosage, period of use, or accumulated dose. Prior to beta-blocker use, there was no significant difference in the incidence of anoxic seizures and oxygen saturation (Table 3). However, in the conditional logistic regression analysis, there was no increase observed in the risk of these factors (Table 4). Only the use of beta-blockers affected low blood glucose.
In hypoglycemic patients, it was shown that infection during infancy triggers a state of poor oral ingestion. Of note, it appears that during infancy, there are many situations that can lead to a state of poor oral ingestion due to infection, which tends to cause hypoglycemic symptoms. In particular, 22q11.2 deletion syndrome and trisomy 21 were found to exhibit susceptibility to infection. However, in our conditional logistic regression analysis, there was no significant difference observed (Table 4).
With regard to heart disease, we only examined tetralogy of Fallot with or without pulmonary atresia but were unable to evaluate the relationship with low blood glucose.
In heart failure, the functioning of glucose-metabolizing enzymes is maintained. However, it has been reported that poor oral ingestion, poor circulation, and the progression of congestive hepatopathy can lead to reduced liver storage of glycogen and, thus, to low blood glucose.23, 25) In the present study, we observed no symptoms of heart failure in the TOFβ+H group. Additionally, BNP levels were not high, and thus, no relationship was observed (Tables 3 and 4).
In hypoxemia patients, it has been reported that tissue hypoxia caused by circulatory insufficiency impairs glucose metabolism in the liver, thereby causing low blood glucose.25) On the other hand, hypoxemia is said to reduce insulin sensitivity.23) In the present study, the TOFβ+H group had significantly higher oxygen saturation than the TOFβ+E group (Table 3). However, the conditional logistic regression analysis did not show a higher risk of low blood glucose (Table 4).
Slender build is considered to be a risk factor for ketotic hypoglycemia22) and is also thought to be a factor that causes low blood glucose. In the present study, in the TOFβ+H group, with a standardized height of −1.57±0.95 SD and standardized body weight of −1.32±0.96 SD, the patients tended to have a slender build, and a Kaup index of 15.2±1.5 indicated a slightly slender build. However, in the TOFβ+E group, the Kaup index was 15.7±1.5, also indicating a slightly slender build, with no significant difference observed (p=0.23). Furthermore, in the conditional logistic regression analysis based on a Kaup index of <16, there was no significant difference observed (Table 4).
2. The mechanism underlying the appearance of beta-blocker-induced hypoglycemia
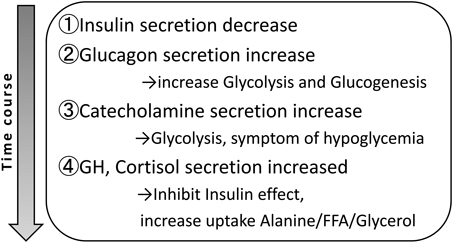
In general, blood glucose level is maintained via a complex mechanism that involves hormones such as insulin and glucagon that target the liver, muscles, and adipose cells as well as enzymes that break down glycogen stored in the liver and substrates supplied from muscle and adipose cells (alanine, fatty acid, and glycerol).26) In particular, insulin increases the uptake of sugars, amino acids, and fatty acids to muscles and adipose cells, thereby lowering blood glucose levels. Glycogen is involved in blood glucose maintenance by utilizing substrates and promoting intrahepatic gluconeogenesis and glycolysis.22, 26, 27)
During hypoglycemic attacks, insulin secretion is first reduced and glycogen levels are increased. Next, catecholamine levels are increased, thereby promoting glycogenolysis and causing the appearance of hypoglycemic symptoms such as palpitations and cold sweats.22) When symptoms are prolonged over a few hours, growth hormones and corticosteroid hormones are activated, which increases catecholamine activity and maintains blood glucose levels.26) In other words, in the mechanism underlying blood glucose maintenance, catecholamine plays a key role in the control mechanism (Fig. 2).27)
During infancy, low blood glucose often presents as ketotic hypoglycemia and excluding endocrine disease, accounts for one-fourth of hypoglycemic cases, with an incidence of 3.9 per 100,000 individuals.20) It has been reported that ketotic hypoglycemia is common among slender individuals. However, in recent years, the catecholamine response to fasting and poor muscle alanine release have been reported.22, 28, 29) Even in ketotic hypoglycemia, catecholamine plays a major role in the control mechanism involved in blood glucose maintenance.
It has been reported that beta-blockers conceal the hypoglycemic symptoms of catecholamines and inhibit glucose-6-phosphatase, insulin, and glucagon, which leads to low blood glucose.30–33) To date, cases of low blood glucose caused by each generation of beta-blockers including carteolol,12, 14, 24) propranolol,5–10) and atenolol11) have been reported. However, the risk of β1-selective and endogenous catecholamine stimulation, as well as the presence or absence of alpha-blocking effects, remains controversial.2, 3, 35–39) In the present study, there were no patients observed to exhibit low blood glucose as a result of beta-blockers other than carteolol. However, the present study was extremely biased in that 97% of all patients were using carteolol; thus, we believe that it cannot be concluded that the level of risk with other agents is high. Furthermore, in the present study, no differences were observed between TOFβ+E and TOFβ+ H groups in terms of dosage, starting time, or completion time, and therefore, the impact on low blood glucose could not be determined (Table 3).
On the basis of the present results, it was inferred that the reason for the high incidence of hypoglycemia in the group using beta-blockers was poor oral ingestion as a result of infection during infancy. However, the use of beta-blockers inactivated the blood glucose maintenance mechanism primarily involving catecholamine, which led to the onset of hypoglycemia.
3. Precautions with Beta-blocker Administration to Infants
When administering beta-blockers to infants, we believe that countermeasures should be adopted for low blood glucose: ① avoiding long-term starvation as per ketotic hypoglycemia, ② frequent ingestion of a high carbohydrate and protein diet, and ③ when fasting is required for performing tests, sugar supplementation should be administered via drip infusion overnight. Furthermore, in such infants, when seizures and disturbance of consciousness are observed, blood glucose levels should be measured.
Limitations to the present study were the small sample size and the fact the study included only 16 hypoglycemic patients, all of whom were found to be in the TOFβ+ group. However, the possibility of hypoglycemic patients in the TOFβ− and PA groups cannot be ruled out. Moreover, the effects of genetic complications and small physical stature on low blood glucose were unclear. This study was conducted at a single center, and we believe that the results could be further clarified by increasing the sample size in future investigations.
In recent years, intracardiac repair for tetralogy of Fallot has been likely to performe during late infancy. However, it has been reported that intracardiac repair performed within 6 months of birth has poor outcomes.40) Anoxic seizures commonly occur within 6 months of birth, and therefore, at our center, we administer prophylactic beta-blockers. On the other hand, there are increased therapies to use beta-blockers such as carvedilol in infants with heart failure. When using beta-blockers in infants, we believe that increased awareness of low blood glucose is needed.
Beta-blockers were used to prevent anoxia in majority of patients with tetralogy of Fallot [214 of 306 patients (69.9%)] and low blood glucose was observed in 16 patients (7.5%). All patients with hypoglycemia were using carteolol. However, in the present study, 97% of patients were using carteolol, indicating a large bias; therefore, we could not conclude that the level of risk associated with other beta-blockers was high. Low blood glucose in patients using beta-blockers was caused by poor oral ingestion due to infection, but other causes could not be identified. The incidence of hypoglycemia in infants using beta-blockers was clearly higher than that in infants in general. Thus, we believe that beta-blocker use is a risk factor for low blood glucose.
Conflicts of Interest
The authors declare that they have no conflict of interest.
引用文献References
1) Cruickshank JM: Beta-blockers and heart failure. Indian Heart J 2010; 62: 101–110
2) Deedwania P: Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: Benefits of vasodilating beta-blockers. J Clin Hypertens 2011; 13: 52–59
3) Fonseca VA: Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin 2010; 26: 615–629
4) Bush GH, Steward DJ: Severe hypoglycaemia associated with preoperative fasting and intraoperative propranolol. A case report and discussion. Paediatr Anaesth 1996; 6: 415–417
5) Chavez H, Ozolins D, Losek JD: Hypoglycemia and propranolol in pediatric behavioral disorders. Pediatrics 1999; 103: 1290–1292
6) Hussain T, Greenhalgh K, McLeod KA: Hypoglycaemic syncope in children secondary to beta-blockers. Arch Dis Child 2009; 94: 968–969
7) Kallen RJ, Mohler JH, Lin HL: Hypoglycemia: A complication of treatment of hypertension with propranolol. Clin Pediatr 1980; 19: 567–568
8) McBride JT, McBride MC, Viles PH: Hypoglycemia associated with propranolol. Pediatrics 1973; 51: 1085–1087
9) Abramson EA, Arky RA, Woeber KA: Effects of propranolol on the hormonal and metabolic responses to insulin-induced hypoglycaemia. Lancet 1966; 24: 1386–1388
10) Hesse B, Pedersen JT: Hypoglycemia after propranolol in children. Acta Med Scand 1973; 193: 551–552
11) Darcie S, Leone CR, Calil VMLT, et al: Glycemia in newborns of hypertensive mothers according to maternal treatment. Rev Hosp Clin Fac Med Sao Paulo 2004; 59: 244–250
12) Sudo A, Ohta Y, Uchida M, et al: Three cases with hypoglycemic convulsion due to administration of carteolol hydrochloride. Jpn J Pediatr 1998; 51: 2335–2338 (in Japanese)
13) Thamer M, Ray NF, Taylor T: Association between antihypertensive drug use and hypoglycemia: A case-control study of diabetic users of insulin or sulfonylureas. Clin Ther 1999; 21: 1387–1400
14) Yamaguchi A, Yamaya T, Kashio Y, et al: Antihypertensive effects of long acting carteolol hydrochloride in patients with diabetic hypertension. Ger Med 1992; 30: 1213–1217 (in Japanese)
15) Infusino F, Pitocco D, Zaccardi F, et al: Low glucose blood levels are associated with abnormal cardiac sympatho-vagal balance in type 2 diabetic patients with coronary artery disease. Eur Rev Med Pharmacol Sci 2010; 14: 203–207
16) Cornblath M, Hawdon JM, Williams AF, et al: Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics 2000; 105: 1141–1145
17) Whipple AO, Frantz VK: Adenoma of islet cells with hyperinsulinism. Ann Surg 1935; 101: 1299–1310
18) Moss and Adams’ Heart Disease in Infants, Children, and Adolescents 7th edition. Lippincott Williams and Wilkins, 2008, pp. 58–59
19) Hano T, Nishio I: Beta-blockades. Jpn J Clin Exper Med 2001: 47–50 (in Japanese)
20) Daly LP, Osterhoudt KC, Weinzimer SA: Presenting features of idiopathic ketotic hypoglycemia. J Emerg Med 2003; 25: 39–43
21) Uchigata Y: Hypoglycemia in neonates. Jpn J Clin Exper Med 2006; Suppl: Endocrine Syndromes III; 181–185 (in Japanese)
22) Yokota Y: Ketotic hypoglycemia in children. Jpn J Clin Exper Med 2006; Suppl: Endocrine Syndromes III; 186–189
23) Hoshino A: Studies on carbohydrate metabolism in infants with congenital heart disease. Part I. Alteration of glucose and lactate metabolism in congenital heart disease. Acta Paediatr Jpn 1981; 23: 162–171
24) Sakata K, Hayashi S, Fukumochi H, et al: Clinical investigation of tetralogy of Fallot patients associated with brain injury: Relevance with beta-blockades (carteolol hydrochloride). J Jpn Pediatr Soc 1991; 95: 2616–2620 (in Japanese)
25) Benzing G, Schubert W, Hug G, et al: Simultaneous hypoglycemia and acute congestive heart failure. Circulation 1969; 10L: 209–216
26) Matsuo N, Tsuchiya H, Cho H, et al: Hypoglycemia. Pediatr Jpn 1982; 23: 1359–1365 (in Japanese)
27) Sasaki H, Yamazaki H: Hypoglycemia die to endocrine disorder. Jpn J Clin Med 2006; Suppl. Endocrine Syndromes III: 211–214
28) Huidekoper HH, Duran M, Turkenburg M, et al: Fasting adaptation in idiopathic ketotic hypoglycemia: A mismatch between glucose production and demand. Eur J Pediatr 2008; 167: 859–865
29) Pershad J, Monroe K, Atchison J: Childhood hypoglycemia in an urban emergency department: Epidemiology and a diagnostic approach to the problem. Pediatr Emerg Care 1998; 14: 268–271
30) Coker RH, Koyama Y, Denny JC, et al: Prevention of overt hypoglycemia during exercise: Stimulation of endogenous glucose production independent of hepatic catecholamine action and changes in pancreatic hormone concentration. Diabetics 2002; 51: 1310–1318
31) Rizza RA, Cryer PE, Haymond MW, et al: Adrenergic mechanisms of catecholamine action on glucose homeostasis in man. Metabolism 1980; 29 Suppl: 1155–1163
32) Cersosimo E, Zaitseva IN, Ajmal M: Effects of beta-adrenergic blockade on hepatic and renal glucose production during hypoglycemia in conscious dogs. Am J Physiol 1998; 275: E792–E797
33) Zderic TW, Schenk S, Davidson CJ, et al: Manipulation of dietary carbohydrate and muscle glycogen affects glucose uptake during exercise when fat oxidation is impaired by beta-adrenergic blockade. Am J Physiol Endocrinol Metab 2004; 287: E1195–E1201
34) Shimizu M, Nakanishi T. Yamamura E. et al: Hypoglycemia in children with chronic heart failure due to carvedilol administration.d Cardiol Card Surg 2004; 20: 432–436 (in Japanese)
35) Jacob S, Rett K, Wicklmayr M, et al: Differential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: The carvedilol-metoprolol study. J Hypertens 1996; 14: 489–494
36) Giugliano D, Acampora R, Marfella R, et al: Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension: A randomized, controlled trial. Ann Intern Med 1997; 126: 955–959
37) Kasanishi K, Uchida S, Kawashima K: Effects of beta-blockades on lgucose metabolism and cardiovascular responce during insulin-induced hypoglycemia. J Clin Therap Med 1987; 3: 1123–1134 (in Japanese)
38) Kasanishi K, Uchida S, Kawashima K: Effects of beta-blockades on lgucose metabolism and cardiovascular responce during insulin-induced hypoglycemia. J Clin Therap Med 1987; 3: 1123–1134 (in Japanese)
39) Ehmer B, Van der Does R, Rudorf J: Influence of carvedilol on blood glucose and glycohaemoglobin A1 in non-insulin-dependent diabetics. Drugs 1988; 36 Suppl: 136–140
40) Masuda M, Kado H, Kajihara N, et al: Early and late results of total correction of congenital cardiac anomalies in infancy. JJTCVS 2001; 49: 497–503