Current Status and Indications of Pediatric Heart Transplant in the United States
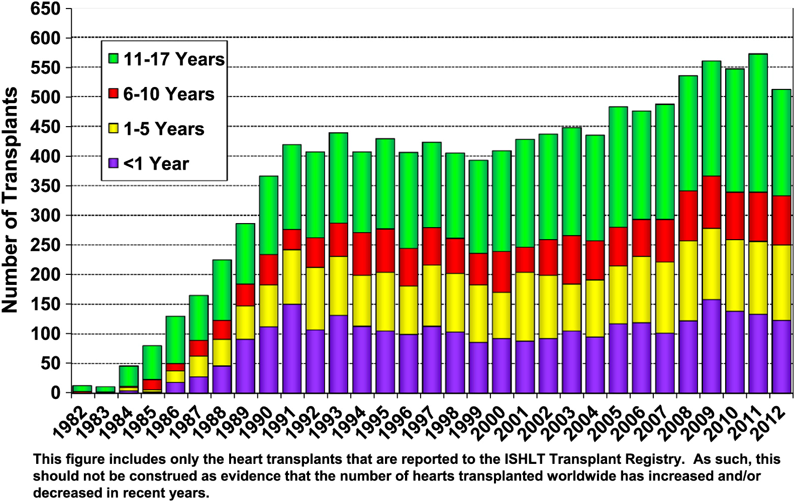
A recent study by Dipchand et al. reported that between 500 and 600 pediatric heart transplants are performed annually in the United States, which is estimated to represent approximately 66% of worldwide cases20) (from International Society for Heart and Lung Transplantation or ISHLT Registry: Fig. 1). Outcomes significantly improved from 1982 to 2011, demonstrating the median survival of 19.7 years for infants, 16.8 years for children from 1 to 5 years, 12.5 years for children from 6 to 10 years, and 12.4 years for children from 11 to 17 years of age at the time of heart transplant.20) For a more recent time period, from 2005 to 2009, the overall survival rates of 91%, 87%, and 83% at 1, 3, and 5 years after transplant, respectively, were reported by Pediatric Heart Transplant Study (PHTS) Registry.21)
Heart transplant should be considered for any patients with medically refractory advanced heart failure. However, in the United States, universal guidelines for pediatric heart transplant have not been adopted, and each transplant program is mandated to develop center-specific criteria.22) A scientific statement published in 2007 by the American Heart Association proposed the indications for heart transplant in pediatric heart disease.23) In this statement, indications were suggested based upon the severity of clinical status graded into 4 different stages (A to D) (Table 1). 24)Class D heart failure is defined as symptomatic heart failure at rest requiring continuous inotrope support, mechanical ventilation, or mechanical device support, which has Class I indication for heart transplant.22) Transplant was also recommended in children with Class C heart failure (present or past history of symptomatic heart failure) who are at risk for sudden death or have pulmonary hypertension.22) However, defining symptomatic heart failure in CHD patients is challenging, as they often accommodate their lifestyle to lower levels of activity gradually, making symptoms more difficult to elicit.22)
Table 1 Proposed heart failure staging for infants and children (International Society for Heart and Lung Transplantation 2004)24)| Stage | Interpretation |
|---|
| A | Patients with increased risk of developing HF, but who have normal cardiac function and no evidence of cardiac chamber volume overload. Examples: previous exposure to cardiotoxic agents, family history of heritable cardiomyopathy, univentricular heart, congenitally corrected transposition of the great arteries. |
| B | Patients with abnormal cardiac morphology or cardiac function, with symptoms of HF, past or present. Examples: aortic insufficiency with LV enlargement, history of anthracycline with decreased LV systolic function. |
| C | Patients with underlying structural or functional heart disease, and past or current symptoms of HF. |
| D | Patients with end-stage HF requiring continuous infusion of inotropic agents, mechanical circulatory support, cardiac transplantation or hospice care. |
| HF: heart failure, LV: left ventricle |
Indications for pediatric heart transplant include CHD, cardiomyopathies (dilated, hypertrophic, and restrictive), and retransplant for graft failure.23) Common considerations for heart transplant in CHD are listed in Table 2.25, 26) Single ventricular lesions are most common (36%), followed by systemic right ventricle (20%).25) Congenital heart disease remains the most common indication for heart transplant in infants (55%) but has decreased over time, whereas cardiomyopathy increased from 35% in the period of 2000 to 2005 to 41% in the most recent era.20) This may be, in part, reflecting the recent decline in the number of primary heart transplant for the hypoplastic left heart syndrome (HLHS) patients and the significant improvement in interstage survival by a staged surgical palliation when compared with the combined wait-list mortality and early post-transplant mortality for HLHS infants.27, 28) There are certain challenges specific to patients with CHD, including increased allosenstization due to prior cardiac operations,7) technical challenges due to cardiac positional anomalies and previous vascular reconstructions, more prolonged intraoperative preparation, and known comorbidities secondary to single ventricular palliation (discussed later).26, 29) Survival following failed single ventricular palliation carries the highest mortality in infants with 1-year survival of 70%,28) compared with 90% to 94% for dilated cardiomyopathy (DCM).30–32)
Table 2 Indications for heart transplant in patients with congenital heart disease23, 25, 26)| 1. SV Physiology | 36% |
| a. Failed SV palliation | |
| b. Failed Fontan | |
| c. Unrepaired HLHS | |
| 2. Systemic RV | |
| a. d-TGA after atrial switch operation (Mustard/Senning) | 12% |
| b. l-TGA (congenitally corrected TGA) | 8% |
| 3. PA/IVS with RVDCC | |
| 4. RVOT lesions (TOF) | 10% |
| 5. LVOT lesions | 8% |
| 6. Neonatal Ebstein anomaly with severe cardiomegaly, severe TR, poor RV function, or sluggish antegrade flow into main PA. | |
| 7. Complex heterotaxy syndrome (with TAPVR and/or severe AVVR) | |
| 8. Others (ASD/VSD/CCAVC) | 27%* |
| The percentages are from 488 patients with CHD who underwent heart transplant.25) *Also includes 3, 6, and 7. SV: single ventricle, HLHS: hypoplastic left heart syndrome, RV: right ventricle, TGA: transposition of the great arteries, PA/IVS: pulmonary atresia with intact ventricular septum, RVDCC: right ventricle-dependent coronary circulation, RVOT: right ventricular outflow tract, TOF: tetralogy of Fallot, LVOT: left ventricular outflow tract, TR: tricuspid regurgitation, PA: pulmonary artery, TAPVR: total anomalous pulmonary venous return, AVVR: atrioventricular valve regurgitation, ASD: atrial septal defect, VSD: ventricular septal defect, and CCAVC: complete common atrioventricular canal. |
Dilated cardiomyopathy is the most common form of cardiomyopathy in children (83% of 1320 patients with cardiomyopathy had DCM listed for heart transplant from 1993 to 2006).33) Seventy-four percent of listed DCM patients ultimately underwent heart transplant, with a 10-year survival rate of 72%.34) Hypertrophic cardiomyopathy (HCM) is an infrequent etiology to be listed for pediatric heart transplant (6% of cardiomyopathies) for which critically ill infants have the highest wait-list mortality (33% within the first year after listing).35) Restrictive cardiomyopathy (RCM) comprises 11% of pediatric cardiomyopathies listed for heart transplant.36) Children with RCM have a generally low wait-list death rate and reasonable overall survival compared with DCM, but this is due, in part, to early listing before they clinically deteriorate, 36) and a much higher proportion of patients with RCM undergo transplant in comparison with other forms of cardiomyopathy.37)
Mechanism of Donor Organ Allocation: The United Network of Organ Sharing (UNOS) System
The allocation of a donor organ to a heart transplant recipient truly provides the gift life to a person with end-stage heart failure. Unfortunately, for many individuals on the transplant list, there is a world-wide shortage of organs. Today in the United States, there are >100,000 patients waiting for organ transplant daily (https://unos.org). Therefore, adherence to the listing and matching processes must be stringently followed to ensure maximal utilization of this precious resource. The United Network of Organ Sharing (UNOS) is a non-profit organization that administers the Organ Procurement and Transplant Network (OPTN) in the United States. UNOS is responsible for managing the national wait-list and matching process, maintaining databases of all organ transplants in the nation, developing policies, monitoring adherence to policies, educating transplant professionals and the public on the benefits of organ donation, and assisting the patient and family during an organ transplant. The matching process considers multiple factors including: age, ability of the patient to recover, ABO status, distance, height and weight, life-support status, and time on the waiting list (https://optn.transplant.hrsa.gov).
In 2016, UNOS updated the pediatric heart transplant allocation policy to ensure maximal utilization of organs (see Table 3). This allows centers to list patients for heart transplant based on the severity of the clinical disease state and risk of death. Once accepted onto a wait list for heart transplant, patients are registered in one centralized national computer that is run by the UNOS Organ Center and links all centers in the United States together. An Organ Procurement Organization (OPO) is a non-profit organization that provides organ recovery services in a geographic region in the United States (https://organdonor.gov/awareness/organizations/local-opo.html). They have coordinators who will conduct a medical and social history to determine suitability of the organ, work with the family and medical staff to discuss the option of organ donation, and manage the informed consent process. Once consent for donation is received, they will manage the clinical care of the donor, enter all donor information into the UNOS computer system, and once a match is made, they will coordinate the timing of the recovery of the organs with the surgical teams and provide follow-up information to the donor family and medical staff on the outcome of the donations. As donor organs are identified, the procuring organization will run a match of potential recipients based on blood type, tissue type, size of the organ, medical urgency of the patient, time on the waiting list, and distance between donor and recipient. The ethnicity, sex, religion, and financial status are not considered as part of the computer matching system. A procurement coordinator then contacts the transplant center for the top-ranked patient, and if the patient is accepted, will coordinate all transportation and surgical timing for harvesting the donor organ. If the organ is declined by the top-ranked patient’s center due to a donor or recipient issue, then the organ would be offered to the next candidate on the match list. Once an organ is accepted, the receiving center will inform the patient and family and coordinate operating room times based on the arrival of the donor heart. Donor hearts are usually best transplanted within less than six hours of ischemic time (https://optn.transplant.hrsa.gov).
Table 3 Pediatric Heart Transplant Listing| Status 1A: A candidate is <18 years old at the time of registration and meets one of the following five criteria below and must be recertified every 14 days: | 1. Requires continuous mechanical ventilation and is admitted to the hospital that registered the candidate. |
| 2. Requires assistance of an intra-aortic balloon pump and is admitted to the hospital that registered the candidate |
| 3. Has ductal dependent pulmonary or systemic circulation, with ductal patency maintained by stent or prostaglandin infusion, and is admitted to the transplant hospital that registered the candidate. |
| 4. Has a hemodynamically significant congenital heart disease diagnosis, requires infusion of multiple intravenous inotropes or a high dose of a single intravenous inotrope, and is admitted to the transplant hospital that registered the candidate. |
| Qualifying Pediatric Status 1A Congenital Heart Disease Diagnoses |
| • Double Outlet Right Ventricle |
| • Atrial isomerism / Heterotaxy |
| • Atrioventricular Septal Defect |
| • Congenitally Corrected Transposition (L-TGA) |
| • Ebstein’s Anomaly |
| • Hypoplastic center Heart Syndrome |
| • Other center Heart Valvar/Structural Hypoplasia |
| • Pulmonary Atresia with Intact Ventricular Septum |
| • Single Ventricle |
| • Tetralogy of Fallot |
| • Transposition of the Great Arteries |
| • Truncus Arteriosus |
| • Ventricular Septal Defect(s) |
| • Other (Specify) |
| Qualifying Pediatric Status 1A Inotropes and Dosages |
| Requires infusion of a single high dose inotrope: |
| • Dobutamine greater than or equal to 7.5 µg/kg/min |
| • Milrinone greater than or equal to 0.50 µg/kg/min |
| • Dopamine greater than or equal to 7.5 µg/kg/min |
| • Epinephrine greater than or equal to 0.02 µg/kg/min |
| ***If the candidate is supported by multiple inotropes, the dosage requirements do not apply. |
| 5. Requires assistance of a mechanical circulatory support device |
| Status 1B: <18 years old and meets one of the following two criteria below and does not require recertification unless the candidates medical status changes: | 1. Requires infusion of one or more inotropic agents but does not qualify for pediatric status 1A. |
| 2. Is <1 year old at the time of the candidates initial registration and has a diagnosis of hypertrophic or restrictive cardiomyopathy. |
| Status 2: <18 year old at the time of registration and not meet the criteria for pediatric status 1A or 1B but is suitable for transplant. No recertification required. | |
| Inactive status: A candidate is temporarily unsuitable for transplant, the candidate will not receive any heart offers during this time. | |
| (https://optn.transplant.hrsa.gov/news/pediatric-heart-allocation-policy-and-system-changes/) |
Although a national system helps maximize the allocation of donor organs, there is still a significant shortage of heart donors. In 2015, there were 644 candidates listed for heart transplant and 460 heart transplants performed in children ages 0–18 years.38) As a temporalizing measure, ventricular assist devices (VADs) have been used as a bridge to transplant in pediatrics, but size limitations and considerable morbidity remain a challenge. Blume et al. recently reviewed 364 patients ages<19 years with VADs, and 80% received left ventricular assist devices, 15% biventricular assist devices, and 2% total artificial hearts, with almost 50% of this cohort surviving to transplant within 6 months but having overall mortality of 19% on device therapy.39) The wait-list mortality is highest for infants <1 year old and in children with CHD who have undergone prior surgical palliation, especially failed Fontan-palliation.26) Consideration should also be given to teenagers with end-stage heart failure who are approaching their 18th birthday since their wait times will be longer after they become 18 years of age. Peng et al. demonstrated that those listed after their 18th birthday waited approximately 8.5 months longer compared with those listed before their 18th birthday due to the competition from adult recipients after age 18 years.40)
Wait-list mortality is a serious problem for infants and children awaiting heart transplant, with the incidence ranging from 13% to 29%5, 41–43); the highest is in children with end-stage CHD.43) This is considered primarily due to absolute donor shortage in relation to the demand and more stringent acceptance criteria of the donor hearts for the patients with CHD because of multiple comorbidities.41, 44) However, questions have also been raised by Almond et al. that the current allocation system may not be structured optimally to reduce transplant mortality, by which an available heart is offered first to the child who has accumulated more status 1A time rather than a child who is likely to die without transplant.42) To reduce the wait-list mortality, certain endeavors have been trialed. The number of ABO incompatible transplants has been significantly increased in recent years with reasonable outcome, especially for infants with higher immune tolerance than older children.45, 46) Donor hearts with diminished ventricular function have been used for pediatric transplant with comparable post-transplant survival when compared with those with normal systolic function.47, 48) Acceptance of a marginal donor heart should be considered in comparison with expected high wait-list mortality.49, 50) On the current allograft allocation system, only 50% of donor hearts were actually used for transplant, and the others were discarded.48) This is, in part, due to poorly standardized current criteria for acceptance of donor hearts.51) A standardized donor scoring system should accurately reflect the likelihood of organ acceptance and predict long-term survival.52) The increased utilization of these unused marginal donor hearts may ameliorate the donor shortage problem and could reduce wait-list deaths.
Immediate Postoperative Management after Heart Transplant
Pediatric heart transplant presents with unique challenges as approximately 40% of heart transplants are performed for the children with advanced heart failure in complex CHD with or without previous surgery.29, 53) In addition to a high wait-list mortality rate in infants awaiting heart transplant,43) early post-transplant mortality is high in infants with CHD, especially those with HLHS.28) Chrisant et al. reported that post-transplant survival of 175 HLHS infants was 72% at 5 years, with 76% of deaths occurring within 3 months.27) However, among the conditional survivors after 1 month of transplant for HLHS, the survival was 92% and 85% at 1 and 5 years, respectively, which is comparable to that for cardiomyopathies,27) suggesting the presence of critical determinants of long term-survival during the early postoperative period.
Transplant for CHD requires special consideration for post-transplant complications, graft survival, and patient mortality, especially in those with single ventricle with previous staged palliation, when compared with transplant for cardiomyopathies. These considerations include prolonged surgical time due to previous cardiac surgery or for anatomical reconstruction and increased risk of allosensitization.54) Prolonged cardiopulmonary bypass (CBP) time and aortic cross-clamp duration are independent predictors of mortality and morbidity after cardiac surgery primarily via increased systemic inflammatory responses, causing multiple organ dysfunction including low cardiac output, prolonged mechanical ventilation, increased pulmonary vascular resistance, excessive bleeding and need for transfusions, acute renal dysfunction, prolonged hospitalization, and in-hospital death.55, 56)
Surgical Procedures for Complex Anatomy
Patients with certain anatomy, including anomalies of the pulmonary or systemic venous return, pulmonary artery distortion, aortic arch anomalies, previous shunts, LeCompte maneuver, and variation of the cardiac position or situs (mesocardia, dextrocardia or situs inversus), usually require significant reconstruction of the venous pathway, aortic arch, and pulmonary arteries. Thus, modification of the donor and recipient procedure is required, and procurement of additional tissue (pulmonary artery, aorta, superior vena cava) with the donor heart provides the best material to facilitate the reconstruction, which may require significant technical expertise and creativity. This added complexity may be regarded as contraindications to heart transplant or may be an incremental risk factor for early mortality. It is now clearly established that there is no anatomical contraindication to heart transplant. With a number of innovative reconstructions, all anatomical abnormalities can be managed using the donor or recipient tissue or both, with excellent outcomes.57, 58)
Bleeding and Vascular Access
Excessive bleeding is a common complication following pediatric heart transplant. The etiology of bleeding complication is often multi-factorial, including prior congenital heart surgery requiring extensive dissection, aggressive anticoagulation strategy, coagulopathy of CPB, and poor preoperative nutritional status.59) Patients who underwent repair or palliation of CHD commonly present with limited vascular access and development of the collateral vessels network. These two conditions can present a formidable challenge in obtaining appropriate lines for infusion of fluids, vasoactive drugs, medications, and future biopsies. In addition, the presence of arterio-venous and/or veno-veno collaterals can be associated with severe blood loss, particularly when extensive dissection is required. In the presence of extensive venous or arterial collateralization, endovascular coiling should be considered prior to transplant.60) This can provide important advantages including decrease in pulmonary venous return during CPB and improvement in cerebral perfusion due to reduced runoff through aorto-pulmonary collaterals. Nevertheless, this strategy may not be feasible in the presence of low systemic oxygen saturation levels pre-transplant. Similarly, closure of residual collaterals should be considered following cardiac transplantation to reduce volume overload on the transplanted heart.
In anticipation for bleeding during sternotomy or difficult dissection, alternative cannulation sites should be considered, including the axillary, femoral, or carotid vessels. Therefore, preoperative knowledge about patency of these vessels is necessary. Acquisition of hemostasis is extremely important during transplant of those individuals who previously had repeated surgery, particularly for those with chronic cyanosis. This cannot be emphasized enough, as in some cases, postoperative hemorrhage has become uncontrollable, leading to death. Pre-emptive measures to mitigate this complication and the appropriate use of targeted blood component therapy guided by timely assessment of clotting activity are extraordinarily valuable.
Allosensitization and Prevention of Acute Graft Failure
Allosensitization, presented as increased panel-reactive antibody (PRA) that measures anti-HLA antibodies, is significantly associated with increased mortality after pediatric heart transplant by eliciting hyperacute rejection and primary organ dysfunction.7, 61) When PRA is higher than 10%, UNOS recommends a prospective crossmatch to lessen the risk of allosensitization. As a result, many patients with elevated PRA may wait longer to receive a negative crossmatch organ, thereby increasing the risk of wait-list mortality.7, 62) Feingold et al. reported their single-center experience that pre-transplant allosensitization was associated with increased incidence of cardiac allograft vasculopathy (CAV), although there was no significant increase in graft or patient survival compared with those in non-sensitized patients.62) On the other hand, an analysis of the UNOS registry database (3,534 patients) demonstrated that PRA >10% was independently associated with worse long-term graft and patient survival after heart transplant.61) A prior sternotomy, possibly a simple marker for a greater exposure to blood products, was associated with increased risk of allosensitization.7) Homograft materials used in prior reparative or palliative surgery are also thought to elicit an immune response in association with an increase in PRA.63, 64) Sensitized post-transplant pediatric patients are considered at high risk for poor outcome.
There is neither a universally accepted therapeutic strategy for achieving desensitization, nor standard methods for measuring the efficacy of the techniques used to achieve densitization preoperatively.6) Consensus statements in 2009 for sensitized patients awaiting transplant suggested the combined use of plasmapheresis, intravenous immunoglobulin (IVIG), and anti-B cell agents (rituximab) to mitigate the development of hyperacute rejection.65) Pollock-BarZib and colleagues reported 1-year survival of 71% for allosensitized patients using aggressive immunosuppression with thymoglobulin induction; tacrolimus; mycophenolate mofetil; and steroids in combination with daily plasmapheresis, IVIG, and rituximab,66) compared with 50% survival reported by Jacobs et al. for sensitized patients.67) Nevertheless, it seems that significant episodes of rejection and development of coronary allograft vasculopathy (CAV) are quite prevalent following initial success with transplant of sensitized recipient, which has led to selective use of desensitization strategies in different centers. To improve outcome following pediatric heart transplant, further research is imperative to establish an optimum immunosuppression regimen to mitigate the effect of allosensitization for patients with end-stage CHD awaiting heart transplant.
Single Ventricle
Single ventricle management requires a surgical staged palliation, in which the initial palliation carries higher mortality, following an intermediate stage and culminating with the Fontan procedure, which currently has minimal operative risk.54) Due to the scarcity of donors and the fragile circulatory physiology among patients with circulations in parallel connected at the arterial level, wait times for a donor heart are long, and wait-list mortality is considerable. During the wait for an organ, it is highly desirable to transition early to a superior cavo-pulmonary connection, with its inherent physiologic advantages and increased circulatory stability.68) In the case of HLHS, heart transplant is associated with excellent outcomes. However, a reduced donor pool has relegated the use of this management strategy only to patients with HLHS and conditions associated with poor outcomes, namely, significant tricuspid regurgitation and decreased ventricular function.27) In these cases, the use of a hybrid palliation as a bridge to transplant has gained increased application, due to the effective palliation achieved with a less-invasive intervention and mitigation of the possibility of sensitization associated with increased use of blood products and homograft for arch reconstruction during the Norwood procedure.54)
Special Consideration for Failed Fontan
Although initial success with single-ventricle palliation achieving the Fontan circulation is high, the Fontan circulation may fail due to primary ventricular dysfunction, usually associated with a normal pulmonary vascular resistance. Additionally, patients may exhibit a failing Fontan physiology with preserved ventricular function but elevation of pulmonary vascular resistance, leading to high pressure in the Fontan pathway, recurrent pleural effusions, chronic protein-losing enteropathy (PLE), ascites, and/or plastic bronchitis.69, 70) Patients are referred for transplantation either due to pump failure or failed Fontan physiology with preserved ventricular function. These two conditions result in different outcomes. While patients with pump failure usually recover promptly and regain their functional capacity, patients with preserved ventricular function have a more protracted course and risk due to a number of associated issues: namely, malnutrition, sensitization, and chronic cyanosis.71) In the recent retrospective European study of 61 patients of failed Fontan who underwent heart transplant (mean age 15.0±9.7 years) from 1991 to 2011, indications were intractable arrhythmia (28%), complex obstruction of Fontan circuit (16%), PLE (23%), impaired ventricular function (31%), and a combination of the above (15%).72)
The outcomes of heart transplant for failed Fontan have been associated with substantial risk and mortality of 24% to 35% until recently,73, 74) with much improved survival in selected centers with significant expertise in the management of this complex patient population.75) Early referral for transplantation, avoidance of long ischemic times, oversizing of the donor, appropriate myocardial protection, meticulous surgical technique for reentry, and reconstruction and acquisition of hemostasis play key roles in a successful outcome.70) More recently and due to the progressive nature of the liver fibrosis and dysfunction associated with a failed Fontan circulation, heart and liver transplantation has been undertaken with good results in a highly specialized center.76)
Management and Long-Term Complications after Heart Transplant in Children
Optimum immunosuppression is essential for long-term graft survival after heart transplant. Most post-transplant complications are caused by under- or over-immunosuppression. Whereas under-immunosuppression is responsible for rejection, over-immunosuppression results in other problems including infection, CAV, post-transplant lymphoproliferative disorders (PTLD), and renal dysfunction.77) Below, we will discuss general guidelines for immunosuppression therapy and these post-transplant complications.
Overall Strategy of Immunosuppression Therapy
The ISHLT guidelines for the care of heart transplant recipients is a complete overview of the evidence-based approach to immunosuppression.78) There are a few common principles utilized in the immunosuppressive regimens administered at most pediatric centers (http://www.uptodate.com/contents/induction-and-maintenance-of-immunosuppressive-therapy-in-cardiac-transplantation), including;
- The highest risk of rejection is early after transplant (within the first 3 to 6 months), for which the most intense immunosuppression should be given (induction) and weaned slowly over the first year.
- All immunosuppressive agents have certain side effects; it may be most prudent to use multiple agents at lower doses to avoid possible drug toxicities.
- Avoid over-immunosuppression, as this may be associated with infections and various forms of malignancy.
Induction is the state of providing intense immunosuppression directly after heart transplant to prevent acute rejection when the immune system is most activated. Induction is usually achieved by administration of anti-thymocyte globulin (ATG)79) or interleukin-2 receptor (IL-2R) antagonists (Basiliximab).80) Utilization of either form of induction helps lessen the need for corticosteroids and calineurin inhibitors (CNI) in the immediate post-operative period. Calcineurin inhibitors are then usually started at 48–72 hours postoperatively when the renal function and urine output are stabilized after surgery. Following transplant, patients usually have a three-drug regimen consisting of a) CNI: tacrolimus or cyclosporine, b) antimetabolite agents: mycophenolate mofetil (MMF) or azathioprine, and c) corticosteroids. Alternatively, mammalian target of rapamycin (mTOR) inhibitors (sirolimus or everolimus) may also be utilized. Immunosuppression in the maintenance phase is focused on providing the lowest dosage of medications to avoid side effects. Most patients are followed on dual therapy with CNI and antimetabolite (tacrolimus/MMF) or CNI and mTOR inhibitors (tacrolimus/sirolimus or everolimus). Corticosteroids are avoided if possible to prevent early-onset diabetes, bone loss, and growth retardation.81, 82) In fact, Auerbach et al. reported no graft survival advantage to using maintenance steroid in pediatric heart transplant recipients.83) Lowering CNI exposure may also help to prevent long-term renal dysfunction, for which mTOR inhibitor (sirolimus) plays a positive role.84, 85) Current trends of immunosuppression therapy in pediatric heart transplant were reviewed elsewhere.86)
Rejection
Following heart transplant, recipients have a life-long threat of rejection, which limits long-term graft survival and endangers patient survival. A recent report demonstrated that 16% of children experienced rejection during the first year post-transplant after discharge (2008 to 2013), a decrease from 27% in the previous era (2004 to 2008).20) Rejection can occur at any time after transplant but may be grouped into three categories: hyperacute, acute, and chronic rejections.78) Hyperacute rejection occurs instantly, within minutes to hours following donor heart reperfusion. Although hyperacute rejection can be severe and even fatal, the incidence has become extremely rare due to the routine use of prospective and virtual cross-match tests.78) Acute rejection starts within the first few weeks post transplant as the immune system gets stimulated directly or indirectly by HLA or non-HLA antigens of the donor heart via acute cellular rejection (ACR) and antibody-mediated rejection (AMR), referring to a response that primarily involves the cell-medicated and humoral arm of the immune system, respectively.87, 88) The recent ISHLT report revealed that the use of induction therapy continues to trend upwards and that most pediatric heart transplant recipients (71%) receive induction therapy of 47% ATG and 25% IL2-R antagonist,89) which is likely responsible for the decline in incidence of rejection.90) However, increased use of induction therapy did not directly influence long-term mortality.90) Chronic rejection typically occurs several years post-transplant and predominantly manifests as CAV leading to graft failure, need for re-transplantation, and/or death. The incidence of late rejection has significantly declined in the recent era, but its effect on mortality and development of CAV has not changed.91) Nonadherence or noncompliance is a known risk factor that is associated with late rejection, especially in adolescents.16, 17, 92)
As clinical manifestation of graft rejection is nonspecific, variable, and unreliable, endomyocardial biopsy (EMB) remains the gold standard for the diagnosis of rejection in cardiac transplant recipients,87) but alternative methods, serum biomarkers and noninvasive image studies, for rejection surveillance have been investigated to overcome the labor-intensiveness, invasiveness, and cost of EMB.93–95) Classical cardiac biomarkers, troponin and brain natriuretic peptide (BNP), have been studied to assess the degree of myocardial damage secondary to graft rejection. The recent study by Patel et al. demonstrated high sensitivity (94%) and high negative predictive value (99%) of cardiac troponin I (cTnI) in detecting acute rejection proven by EMB in 98 adult heart transplant recipients.96) On the other hand, the reliability of BNP for rejection surveillance has not been proven.94, 97) Other novel investigative serum biomarkers have been proposed as tools for rejection surveillance. Quantification genotyping of circulating donor-specific cell-free DNA, a marker for cellular injury caused by rejection, has been proposed as a sensitive, noninvasive method to detect rejection.98, 99) A group of French researchers recently demonstrated differential expression of microRNAs (miRNAs), miR-10a, miR-155, miR-31, and miR-92, both in tissue and serum, that indicates allograft rejection with high accuracy.100)
Noninvasive imaging studies have been investigated for possible diagnostic tools for identifying acute rejection and early graft failure.93, 101) Main features of early graft failure are LV or biventricular dysfunction with hypotension, low cardiac output, and high filling pressure, which can be detected by conventional echocardiogram, but most cases of acute rejection are diagnosed by surveillance EMB even if the patient is asymptomatic with normal LV systolic function.101) Flanagan et al. reported an increase of LV myocardial performance index (MPI) in 40 children with acute cellular rejection compared with 40 control patients without rejection after heart transplant.102) Tissue Doppler imaging (TDI) was assessed in 122 pediatric heart transplant recipients in which significant decline in biventricular TDI velocities were noted during rejection from the baseline. With frequent routine assessment, they proposed an absence of TDI velocities changes from the baseline as a reliable marker for freedom from rejection.103) Global longitudinal peak systolic strain (GLS) obtained by speckle-tracking echocardiography has been suggested as a suitable parameter to detect subclinical allograft dysfunction.101) However, others argued that there were no differences in speckle-tracking measures between transplant patients with rejection and those without.104) At this point, there is no single echocardiographic parameter to sufficiently replace EMB in identifying graft rejection. Butler et al. recently reported the effectiveness of cardiac magnetic resonance imaging (CMRI) as a possible screening method for rejection by demonstrating high sensitivity (93%) and high negative predictive value (98%) in predicting biopsy-positive heart transplant rejection with quantifying myocardial edema (T2 relaxation time) and right ventricular volume index.105) Further investigations are required for establishing noninvasive imaging studies to reliably diagnose acute graft rejection in both symptomatic and asymptomatic patients.
Infections
A timeline of infections after solid organ transplant is generally outlined into 3 phases: within 1 month, at 1 to 6 months, and after more than 6 months.106) More than 90% of infections occurring in the first month are nosocomial bacterial and candida infections of the surgical wound, lungs, urinary tract, or vascular access device. From 1 to 6 months after transplant, the immunomodulating viruses, particularly cytomegalovirus (CMV) and Epstein-Barr virus (EBV), begin to exert clinically significant effects in combination with sustained immunosuppression. Six months after transplant, more than 80% of patients are in stable condition with minimal long-term immunosuppression therapy with good allograft function. Approximately 10% of patients have chronic or progressive infection with hepatitis type B (HBV), hepatitis type C (HVC), CMV, EBV, or papilloma virus.106)
According to a PTHS study database of 2113 transplanted children, infection was a second-most frequent cause of death among 390 post-transplant deaths (rejection 18%, infection 12%, early graft failure 10%, sudden cardiac death 9%, and CAV-related 8%).21) Infection is the most common cause of unexpected hospitalization, particularly during the first transplant year.107) Intensive immunosuppression to prevent acute and chronic graft rejection inevitably causes increased susceptibility to various infections. George et al. demonstrated that adolescents are much more at risk of death from rejection, whereas elderly recipients are at high risk of infectious death, suggesting the inverse relationship between risk of rejection and that of infection among transplant recipients.8) Infant recipients were more vulnerable to more severe form of infections and more chronic/recurrent illness when compared with older children108). In 4458 pediatric heart transplant recipients, 81% developed some type of infection that required hospitalization or intravenous therapy, in which bacterial infection were the most commonly identified pathogens (43%), followed by virus (31%), and fungi (6%).109) Unlike in adults, the most common site of bacterial infections was the bloodstream (25%) in children, followed by pulmonary (21%), gastrointestinal tract (9%), and urinary tract (9%) with overall mortality of 34% during the observation period.109) Risk factors for infectious mortality in pediatric heart transplant recipients include diagnosis of congenital heart disease, pre-transplant ECMO, cardiac reoperation before discharge, pre-transplant infection requiring antibiotics, and pre-transplant creatinine elevation.110) Young children who underwent heart transplant at age less than 2 years are particularly vulnerable to invasive pulmonary infection, pneumonia, and bronchiestasia due to Streptococcus pneumoniae because of failure to produce effective antibodies against capsular polysaccharide.111, 112) Pneumococcal vaccination is highly recommended for transplant patients.
Cytomagalovirus infection is especially significant, as it not only causes direct infections but also modulates the host immune system to induce acute and chronic rejections including CAV.113) In a prospective study of 378 adults after heart transplant in a single center, nearly half of CMV infections occurred within the first 2 months after transplant, and the use of everolimus significantly lowered the rate of CMV infection; no difference was observed between cyclosporine A and tacrolimus-treated patients.114) Chronic infection by EBV elevated risk of PTLD (discussed later).
Invasive fungal infection (IFI) occurs frequently within the first 3 months after heart transplant, largely reflecting early nosocomial Candida and Aspergillus infections via the surgical site.115) Patients requiring additional induction immunosuppression or delayed chest closure are at increased risk of invasive fungal infection. Systematic surveillance of these infections and timely initiation of pre-emptive treatment in addition to prophylactic treatment are imperative to prevent infectious complications. In children, IFI occurs approximately 7% of total post-transplant infection, associated with 49% mortality rate within 6 months after transplant.116) Candida and Aspergillus species made up the majority of fungal infections (66% and 16%, respectively). Risk and mortality are highest in the first 6 months post-transplant especially in those with previous cardiac surgery and those requiring mechanical supports including ECMO, VAD, and mechanical ventilation.116)
Cardiac Allograft Vasculopathy
Cardiac allograft vasculopathy is a major cause of late heart graft failure, retransplant, and death that occurs in approximately 25% to 34% of pediatric patients within 10 years of transplant.89, 117, 118) The incidence of CAV is higher in adults, in which more than 50% of graft recipients develop clinically significant CAV within 10 years after transplant.119) Diffuse stenoses due to concentric intimal expansion and inadequate compensatory outward remodeling in both epicardial and intramyocardial arteries result in tissue malperfusion, ischemic injury, and graft loss.9) Morphological manifestations of CAV are diverse vascular narrowing, consisting of intimal fibromuscular hyperplasia, atherosclerosis, and inflammation (vasculitis) in the advantitia with relative preservation of the muscular media.120, 121) Clinical symptoms of myocardial ischemia caused by CAV are either atypical or variable due to absent or partial reinervation of the donor hearts. Price et al. reported their institutional experience of 66 post-transplant children in which 27 (41%) developed CAV over the 16-year period. Of 22 patients with the symptom complex of abdominal, chest, and/or arm pain, 18 (82%) were found to have CAV.122) Sudden death or resuscitated sudden death occurred in 15 (68%) of 22 patients with the symptom complex.122) Despite clinically silent progression and lack of symptoms, early detection of CAV is essential to minimize this life-threatening complication. Although angiography is regarded as a gold standard to make a diagnosis of CAV,123) the confluent nature of vascular narrowing has made its early identification difficult. No ideal modality for surveillance exists at the present time, but some diagnostic modalities have been studied including intravascular ultrasound (IVUS) and dobutamine stress echocardiography (DSE).121, 124) Hemodynamic abnormalities, especially restrictive ventricular physiology and decreased systolic function, were shown to be associated with the development of CAV125–127) and may be helpful in detecting early phase of CAV. Multiple serum markers have been studied for their correlation to the development of CAV, but none of them are specific to CAV.128) Recently, a circulating microRNA, miR-628-5b, was reported as a promising serum biomarker for advanced CAV in adults.129)
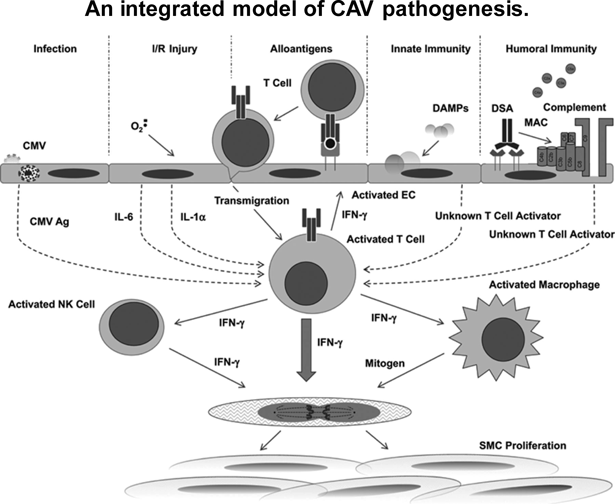
Understanding of the pathogenesis has been limited in part due to lack of relevant animal models; there are profound differences in how rodents and humans respond to allogenic blood vessels.9, 130) Pathogenesis of CAV consists of complex interactions among multiple factors including infection, ischemia/reperfusioin (I/R) injury, alloantigens, innate immunity, and humoral immunity (Fig. 2), but the main pathological process stems from the consequences of chromic alloimmunity, a finding supported by a study including the involvement of host T cells and B cells, lesions restricted to graft vasculature, lack of over-graft cell lysis, and chronic time course over month to years.131) The chronic alloimmunity involves both (a) innate immune cells, including natural killer (NK) cells and macrophages, and (b) adaptive immune cells including host-derived T cells, B cells, and macrophages.9) Data from animal models and human tissues suggest that CAV is represented by a form of host T cell-mediated delayed type hypersensitivity (DTH) via enhanced production of interferon-γ (IFN-γ), which stimulates proliferation of host smooth muscle cells in the intima.131) Donor-specific antibody (DSA), typically against HLA-DQ molecule expressed by graft endothelial cells, may be responsible for adventitial inflammation and increases a risk of developing CAV.132) Other pathogenetic factors include perioperative ischemia-reperfusion injury with endothelial dysfunction,133–135) conventional risk factors of atherosclerosis,136) CMV infection,137) and genetic predisposition and polymorphism138, 139) (reviewed in Merola et al.,9) Schumacher et al.,10) and Pober et al.131)). Reduced bioavailability of nitric oxide (NO) due to endothelial dysfunction is recognized as contributing to the development of intimal thickening in mice and humans.140, 141)
There is no proven medical intervention to prevent CAV or to reverse established disease; potential prevention of CAV depends primarily on the understanding of underlying molecular and cellular mechanisms.124) Modifications in immunosuppressive therapies have been proposed to reduce the risk of CAV. Newly introduced mammalian targets of rapamycin (mTOR) inhibitors, sirolimus and everolimus, have been shown to attenuate the development of CAV.142) Further basic research in understanding pathogenesis of CAV should be encouraged for better management for this life-threatening condition.
Post-transplant Lymphoproliferative Disorders
Post-transplant lymphoproliferative disorders (PTLD) encompass a broad spectrum of lymphoid disorders that share qualities of both infection and malignant disease, ranging from benign polyclonal hyperplasia to malignant monoclonal neoplasms (lymphoma).143) They are most often B-cell origin and commonly contain EBV.143) Webber and his colleagues reported the data of a multicenter study from the PHTS in which 5% of 1184 primary transplant recipients (6.1±5.7 years old) developed PTLD at 23.9 ±20 months after heart transplant.144) The most common sites were gastrointestinal (39%), lung or airway (25%), and cervical adenopathy (18%). At 3 years after diagnosis, only two-thirds of patients were alive, with about one-fourth of patients dying within the first year after diagnosis.144) Manlhiot et al. reported that 13% of 173 post-transplant children (median 4.1 years) developed PTLD at a median age of 7 years (10 months to 16 years). Freedom of death or disease relapse was 72%, 58%, and 30% at 1, 3, and 5 years after diagnosis, respectively.11)
Because of its rapid progression with high morbidity and mortality after diagnosis, early recognition of PTLD is critical. Prolonged constitutional symptoms, including persistent fever, diaphoresis, and/or weight loss in association with localized symptoms (mainly respiratory and gastrointestinal) should raise alarm for possible PTLD.12, 145) A multidisciplinary approach among the pediatric transplant cardiologist, oncologist, radiologist, and pathologist is warranted to make a prompt and accurate diagnosis of PTLD. Risk stratification with EBV status, i.e., transplant of EBV(+) donors into EBV(−) recipients, elevated EBV load in the peripheral blood, increased dose of ATG, and transplant age have been proposed as important variables to predict PTLD.146, 147) For treatment, reduction of immunosuppression (RI), a 50% to 75% dose reduction, is advised as an initial therapeutic approach, especially for early PTLD. However, RI by itself may lead to potential rejection, as 61% (19 of 31) developed rebound acute cellular rejection during the first 6 months after diagnosis of polymorphic PTLD.144) The use of mTOR inhibitors and antimetabolites (azathioprine and mycophenolate mofetil) allows concomitant reduction of CNI, but their clinical efficacy in suppressing PTLD needs to be further investigated.148) Low-dose cytotoxic chemotherapy may be introduced in combination with rituximab and anti-B cell antibody with reasonable outcome.149)
Renal Dysfunction
Renal dysfunction or acute kidney injury (AKI) is a common complication before and after heart transplant; 73% of heart transplant recipients develop AKI postoperatively.150) Renal dysfunction is frequently seen in advanced heart failure, whereas myocardial dysfunction is induced by worsening renal failure, suggesting bidirectional pathological interaction between heart and kidney to deteriorate circulatory homeostasis (cardio-renal syndrome).151, 152) Post-transplant renal dysfunction comprises 1) pre-transplant baseline renal dysfunction associated with advanced heart failure,153) 2) AKI following cardiac surgery (especially, ischemia-reperfusion injury and low cardiac output),154) and 3) AKI and chronic kidney disease (CKD) due to nephrotoxic medications, especially CNI (cyclosporine and tacrolimus).155) On the contrary, Gupta et al. reported the some improvement of renal function in the acute phase (up to 20 days) after transplant in the majority of patients regardless of age and the underlying cardiac diagnosis, suggesting a certain positive effect of heart transplant in mitigating worsening of renal function.156) In a small institutional study, Chinnock et al. reported that the immunotherapy with sirolimus with reduced CNI improved renal function without increasing risk of rejection.84)
The relationship between AKI and CKD was studied by Hollander et al., who demonstrated that non-recovery from AKI was likely due to more advanced renal injury during an acute phase and was associated with the development of CKD within the first year.155) Post-transplant recipients with chronic renal insufficiency (CRI), defined as serum creatinine >2.5 mg/dL, were seen in 4% of patients at 5 years and nearly 12% of patients at 10 years, and were shown to have 9-fold increased risk of death when compared with patients without (CRI).157) Patients with late renal dysfunction demonstrated continued decline in renal function, and decreased estimated glomerular filtration rate (eGFR) at one year post-transplant was shown to predict late onset of renal dysfunction.13) Careful surveillance of these clinical markers is essential to identify early stage of CKD.