Tetralogy of Fallot (TOF) describes a well-known combination of four cardiac, congenital morphological features due to a single developmental defect: an anterior deviation of the septal insertion of the infundibular septum from its usual position in the normal heart.1, 2) It is widely recognized that this causes malalignment of the outlet septum with the rest of the septum leading to the septally defective aorta connecting to both ventricles and a narrowed subpulmonary infundibulum.3, 4) In spite of numerous reviews and studies,5–8) however, the question of the frontal architecture around the right ventricular outflow tract of this morphological abnormality has yet to be investigated.
Reconstruction of the narrowed right ventricular outflow tract is an important element for total correction of TOF. Reconstruction without stenosis and regurgitation provides unrestricted blood flow and preserves right ventricular function that leads to better quality of life and prognosis. Even though creation of the right ventricular outflow tract itself is a prime concern for any surgeon, however, the anterior structure surrounding this reconstruction receives only secondary consideration. As the optimal age for repair decreases within the infant stage,9, 10) it becomes much more critical for reconstruction to maintain function for the longer periods of an expected lifespan.11, 12) It therefore behooves the surgeon to deeply consider any subsequent structural changes in the anterior area of the outflow tract to optimize the surgical approach for maximum quality.
We hypothesized that blood flow pressure on the right ventricular outflow tract (which peak pressure is usually as similar high as that of left ventricle due to large VSD) could cause an anterior wall prominence together with anteriorly deviated septum from behind compared to a normal heart. If this phenomenon is not related to the defect, however, the surgical intervention could then take it into account when planning the anterior expansion of the stenotic outflow tract. An understanding of this detailed mechanism and its implications would therefore help us to realize an ideal surgical repair for the tetralogy of Fallot.
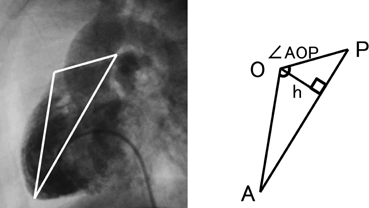
We used right ventriculography on cardiac catheterization to visualize and plan surgical intervention as well as establish a baseline for the anatomy of the front face of the right ventricular outflow tract.
We reviewed 51 cases of tetralogy of Fallot from Ibaraki Children’s Hospital from March 1996 to December 2014. Cases with comorbidity of atrioventricular septal defects, pulmonary atresia, and absent pulmonary valves were excluded. We also reviewed 34 cases of isolated ventricular septal effects without pulmonary hypertension during the same period, whose right ventricular outflow tract structure was considered to be nonspecific. None of the cases had been surgically treated until the cardiac catheterization in this study.
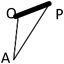
We applied a geometric method to clarify the morphology of the right ventricular outflow tract. Using the lateral view of right ventriculography on cardiac catheterization, we defined 3 fixed points on the anterior face from the right ventricular apex through to the main pulmonary artery at the timing of end-diastolic phase. Point “A” was on the apex of right ventricle and point “P” was on the main pulmonary artery at the right/left bifurcation. These two fixed flow points are not affected by the degree of anterior prominence of the right ventricular outflow tract. Point “O” was the most prominent point which could get maximum distance from AP on the right ventricular outflow tract (Fig. 1). We made geometric measurements on a triangle defined as AOP. The length of AP, AO, and OP were measured. Also the height “h” of the triangle from base AP to point O, and the angle “∠AOP” was measured as the value representing the degree of anterior prominence of the right ventricular outflow tract. Similar measurements were done on VSD hearts. The results were compared between the tetralogy of Fallot (TOF group, n=51) and ventricular septal defect groups (VSD group, n=34).
All quantitative values were summarized as the median and range. Comparisons between the groups were done using the Mann–Whitney U test. All reported p values are 2-sided and the level of significance was 0.05. Statistical analysis was performed using SPSS statistical software (version 22, IBM SPSS Inc., Armonk, NY).
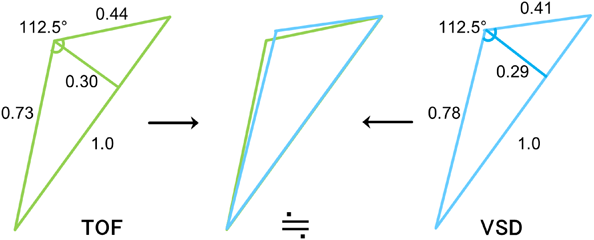
Median age at the study was 362 days in the TOF group (range, 47–1157 days), and 237days in the VSD group (range, 47–2533 days). Height and body weight of each group was 71.0 cm (range 50.2–84.2 cm) and 8.5 kg (range 2.8–12.2 kg) in the TOF group, 69.4 cm (range 55.3–114.0 cm) and 7.9 kg (range 4.1–21.3 kg) in the VSD group (Table 1). These differences were not found to be significant. Actual length of AP, which stands for the distance of a straight line from the apex of right ventricular to the main pulmonary artery at bifurcation, was 60.3 mm (range 44.3–84.5 mm) in the TOF group, which was significantly shorter (p<0.001) than the 63.7 mm (range 26.3–99.9 mm) measurement in the VSD group. Length of AO, which stands for the distance of a straight line from the apex to the most prominent point of right ventricular outflow tract, was 44.4 mm (range 28.7–62.0 mm) in the TOF group which was shorter (p<0.001) than the 51.3 mm (range 22.1–84.4 mm) in the VSD group. The quantity of the differences between the groups was very small, however they were obvious. Length of OP, which stands for the distance of a straight line from the most prominent point of right ventricular outflow tract to pulmonary bifurcation, was 26.3 mm (range 15.9–40.9 mm) in the TOF group and 28.3 mm (8.9–37.5 mm) in the VSD group and no significance was found in this difference (Table 2). Scatter diagrams of actual values of the AOP triangle of both groups are shown in Fig 2. Since the length of AP, which is the base of triangle AOP, differs in each group, we utilized the length of AO and OP proportional to AP to compare the geometric shape itself of the AOP triangle between the groups. Points A and P were the two fixed points which we defined as a point which would not be affected by the degree of anterior prominence of the right ventricular outflow tract. Relative length of AO for AP (relative AO=AO/AP) was 0.73 (range 0.56–0.91) in the TOF group and this was significantly smaller (p=0.004) than the 0.78 (range 0.70–0.86) value in the VSD group. Relative length of OP for AP (relative OP=OP/AP) was 0.44 (range 0.33–0.59) in the TOF group which was longer (p<0.001) than the 0.41 (range 0.28–0.48) result in the VSD group (Table 3). We also investigated the height h and angle ∠AOP to determine how prominent the right ventricular outflow tract is in the tetralogy of Fallot. Relative length of h for AP (relative h=h/AP) was 0.30 (range 0.19–0.41) in the TOF group and 0.29 (range 0.21–0.36) in the VSD group which was not found to be significant. Angle ∠AOP in the TOF group was 112.5° (range 96.2–134.8°) and 112.5°(range 98.6–126.3°) in the VSD groups; this difference, even though the ranges varied, was not found to be significant (Table 3). Fig. 3 shows the complete schema of triangles representing the architecture of the right ventricular outflow tract in both the TOF and VSD groups. The difference in geometric shapes of triangles between the groups was insignificant.
Table 1 Clinical characteristics of patients | TOF group (n=51) | VSD group (n=34) | p Value |
|---|
| Age, days (range) | 362 (47–1157) | 237 (47–2533) | 0.43 |
| Height, cm (range) | 71.0 (50.2–84.2) | 69.4 (55.3–114.0) | 0.62 |
| Weight, kg (range) | 8.5 (2.8–12.2) | 7.9 (4.1–21.3) | 0.60 |
| TOF tetralogy of Fallot, VSD ventricular septal defect |
Table 2 Actual length of 3 sides of triangle AOP | TOF group (n=51) | VSD group (n=34) | p Value |
|---|
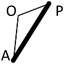 | AP, mm | 60.3 (44.3–84.5) | 63.7 (26.3–99.9) | <0.001 |
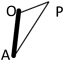 | AO, mm | 44.4 (28.7–62.0) | 51.3 (22.1–84.4) | <0.001 |
 | OP, mm | 26.3 (15.9–40.9) | 28.3 (8.9–37.5) | 0.17 |
Table 3 Relative lengths for AP of each side and height of triangle AOP, and angle ∠AOP | TOF group (n=51) | VSD group (n=34) | p Value |
|---|
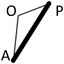 | Relative AP=AP/AP | 1.0 | 1.0 | na |
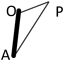 | Relative AO=AO/AP | 0.73 (0.56–0.91) | 0.78 (0.70–0.86) | 0.004 |
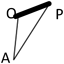 | Relative OP=OP/AP | 0.44 (0.33–0.59) | 0.41 (0.28–0.48) | <0.001 |
| TOF group (n=51) | VSD group (n=34) | p Value |
|---|
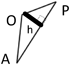 | Relative h=h/AP | 0.30 (0.19–0.41) | 0.29 (0.21–0.36) | 0.35 |
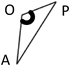 | Angle ∠AOP° | 112.5 (96.2–134.8) | 112.5 (98.6–126.3) | 0.44 |
The actual length of AP and AO were significantly shorter in the TOF group. Since measurements in this study were from ventriculography, results would therefore reflect the length of the endocardial side of the heart. In this case, afferent hypertrophy of the right ventricle due to TOF might be the reason for the shortened AP and AO. However, after geometric comparison of triangle AOP between the groups, we found only very small visible differences in the overall shapes of the triangles. The relative heights of anterior prominence of the right ventricular outflow tract were nearly the same in both groups. ∠AOP, which, to date, has not been reported in previous studies, was identified as 112.5°in both groups. Taken together, this does not support the hypothesis that the tetralogy of Fallot affects the anterior architecture of the right ventricular outflow tract.
Therefore, our sub-hypothesis that blood flow through a narrowed right ventricular outflow tract caused by an anteriorly deviated infundibular septum could cause the right ventricular outflow tract to develop anterior prominence was also not supported. One possible reason for the failure of the hypothesis could be due to load fluctuation: TOF causes the right ventricle to develop two outputs, one being the narrowed tract to the pulmonary artery and the other to the overriding aorta. In this case, flow direction is decided by afterload resistance and blood flow passing through the right ventricle may find easier drainage into the overriding aorta instead of pushing the anterior wall of the right ventricular outflow tract forward. Also, the degree of deviation in the anterior infundibular septum directly translates into narrowing of the outflow tract to become totally obstructed (i.e., tetralogy of Fallot with pulmonary atresia) when the degree of anterior deviation is severe. From our study, we could not point out specific anterior features of the right ventricular outflow tract in the tetralogy of Fallot. From the morphological literatures and textbooks, degree of infundibular deviation related to the degree of infundibular stenosis. There is a possibility that the nonspecific features in this area may be adjunctive mechanisms of infundibular stenosis combined with an anteriorly deviated infundibular septum.
There are several limitations in this study. Since measurements were from ventriculography, results would reflect only the endocardial side of the heart, which may slightly differ from the real morphology of the heart. Also, as all ventriculography measurements were done using a lateral side view, the rotation of the heart in each individual was not considered. This may factor in to loss of precision in measurements.
In conclusion, an anteriorly deviated infundibular septum which narrows the right ventricular outflow tract (the essence of morphological feature of TOF) has no significant correlation to the anterior wall of the right ventricular outflow tract. Furthermore, the anterior profile of the right ventricular outflow tract in TOF is nonspecific. Surgeons should be aware of this morphological feature and expand the anterior wall of the right ventricular outflow tract forward in conformity to the anteriorly deviated infundibular septum to restore proper blood flow passage.
謝辞Acknowledgments
The authors wish to thank Bryan J. Mathis for language direction.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the institutional review board of the Ibaraki Children’s Hospital (IRB approval No. 29IRB-1).
Informed consent: For this type of study formal consent is not required.
引用文献References
1) Anderson RH, Jacobs ML: The anatomy of tetralogy of Fallot with pulmonary stenosis. Cardiol Young 2008; 18 Suppl 3: 12–21
2) Kirklin/Barratt-boyes: Cardiac Surgery 4th edn. Philadelpia, Elsevier, 2013, pp1362–1374
3) Becker AE, Anderson RH: Pathology of Congenital Heart Disease, London, Butterworths, 1981, pp191–198
4) Anderson RH, Becker AE: The Heart. London, Gower Medical Publishing, 1992, pp7.7–7.10
5) Goor DA, Lillehei CW, Edwards JE: Ventricular septal defects and pulmonic stenosis with and without dextropoition. Anatomic features and embryologic implications. Chest 1971; 60: 117–128
6) Becker AE, Connor M, Anderson RH: Tetralogy of Fallot: A morphometric and geometric study. Am J Cardiol 1975; 35: 402–412
7) Anderson RH, Allwork SP, Ho SY, et al: Surgical anatomy of Tetralogy of Fallot. J Thorac Cardiovasc Surg 1981; 81: 887–896
8) Howell CE, Ho SY, Anderson RH, et al: Variations within the fibrous skeleton and ventricular outflow tracts in tetralogy of Fallot. Ann Thorac Surg 1990; 50: 450–457
9) Van Arsdell GS, Maharaj GS, Tom J, et al: What is the optimal age for repair of tetralogy of Fallot? Circulation 2000; 102 Suppl III: III-123–III-129
10) Al Habib HF, Jacobs JP, Mavroudis C, et al: Contemporary patterns of management of tetralogy of Fallot: Data from the society of thoracic surgeons database. Ann Thorac Surg 2010; 90: 813–820, discussion, 819–820
11) Tweddell JS, Simpson P, Li SH, et al: Timing and technique of pulmonary valve replacement in the patient with tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2012; 15: 27–33
12) Fuller S: Tetralogy of Fallot and pulmonary valve replacement: Timing and techniques in the asymptomatic patient. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2014; 17: 30–37