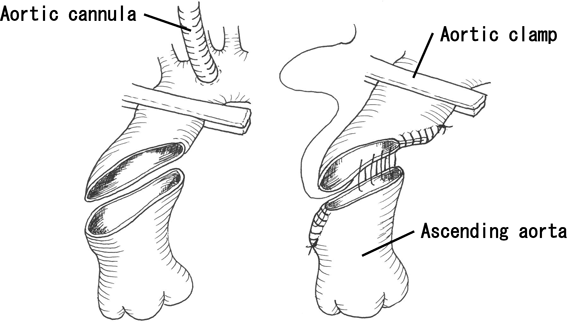
The most important aspect of the aortic extension technique using an autologous aortic tissue is as follows. An arterial cannula was inserted into the peripheral portion of the ascending aorta or aortic arch. Cardiac arrest was achieved via cardioplegia after crossclamping. The clamp was applied from the peripheral ascending aorta to the inner curvature of the aortic arch. The middle of the ascending aorta was obliquely transected from just above the sinotubular junction on the greater curvature side to the opposite side of the brachiocephalic artery origin on the lesser curvature side. The lesser curvature side of the distal stump of the ascending aorta was plicated, thereby reducing its diameter to near-normal size. The outer curvature side of the proximal stump was similarly plicated. Once the anastomotic orifice was reduced in size, both stumps were anastomosed (Fig. 1). This made it possible to reduce the diameter of the ascending aorta, and the ascending aorta could then be extended outward to coincide with the displacement of the anastomotic orifice, thereby enabling the enlargement of the retroaortic space.3)
This aortic extension technique was performed for five patients from 2005 to 2013, and we examined the mid-term results in these patients.
The main diagnoses for the five patients were a double outlet right ventricle with multiple defects in the muscular ventricular septum in one patient and single ventricle in three patients, implying that a single ventricle repair was required for all four of these patients. The other patient required biventricular repair for pulmonary atresia and a ventricular septal defect (VSD) accompanied by major aortopulmonary collateral artery (Table 1). All patients initially presented with anatomical atresia of the main pulmonary artery and dilatation of the ascending aorta. Additional observations included nonconfluence of the left and right pulmonary arteries (n=3), aneurysmal enlargement of the ascending aorta (n=2), and tracheal stenosis (n=1).
Table 1 Preoperative diagnosis| No. | Diagnosis | Pulmonary artery | Ascending aorta | Tracheal stenosis | Previous operation |
|---|
| 1 | DORV, multiple VSD | atresia, non-confluent | dilatation | — | rt.BTS, lt.BTS, BDG, PA plasty |
| 2 | SV | atresia | aneurysm | — | lt.BTS |
| 3 | SV | atresia, non-confluent | dilatation | yes | lt.BTS, rt.BTS |
| 4 | SV, TAPVC | atresia, non-confluent | aneurysm | — | lt.BTS, rt.BTS, TAPVC repair, PA plasty, BDG |
| 5 | VSD, MAPCA | atresia | dilatation | — | central shunt, UF, palliative RVOTR |
| BDG: bidirectional Glenn, BTS: Blalock-Taussig shunt, DORV: double outlet right ventricle, MAPCA: major aorto-pulmonary collateral artery, PA: pulmonary artery, RVOTR: right ventricular outflow tract repair, SV: single ventricle, TAPVC: total anomalous pulmonary venous connection, UF: unifocalization, VSD: ventricular septal defect |
Similar to the previous surgery, pulmonary artery shunt was performed once or twice in all patients. In addition, bidirectional Glenn procedure (n=2), repair of the total anomalous pulmonary venous return (n=1), and pulmonary artery unifocalization, as well as palliative right ventricular outflow tract reconstruction (n=1), were performed.
In our patients, age (in months) at surgery; body weight; and operative data such as aortic cross-clamp time; pre- and postoperative morphology of the aortic arch using three-dimensional -computed tomography (3D-CT); and long-term prognosis were assessed. Furthermore, the preoperative ratio of the maximum to peripheral diameter of the ascending aorta and the postoperative ratio of the suture site to the peripheral diameter were measured using aortic angiography. Then, the pre- and postoperative measurements were compared and the differences were assessed for significance using paired t-test.
The mean patient age at the time of surgery was 18.2±7.6 months (range, from 7 months to 2 years), and the mean body weight was 8.4±0.9 (range, 7.1–10.0) kg (Table 2). Simultaneous surgeries included total cavopulmonary connection (TCPC)-type Fontan surgery (n=2), pulmonary angioplasty (n=2), bidirectional Glenn procedure (n=1), the Rastelli procedure (n=1), aortic valvuloplasty (n=1), and aortopexy (n=1). The mean duration of aortic clamping was 64.8±28.6 (range, 32–112) min. Additional surgical procedures that were simultaneously performed under cardiac arrest included pulmonary angioplasty (n=2), TCPC with anastomosis to the pulmonary side(n=1), TCPC with anastomosis of the inferior vena cava side (n=1), and VSD closure (n=1). In all patients, the aortic extension procedure was completed in approximately 30 min.
Table 2 Patient demographics and operative data| No. | Age (month) | Body weight (kg) | Concomitant procedure | Procedure during aortic cross-clamp | Aortic cross-clamp time (min) | Follow-up (month) |
|---|
| 1 | 25 | 8.2 | TCPC | Aortic extension+anastomosis of PA side | 55 | 130 |
| 2 | 7 | 8.3 | BDG | Aortic extension | 32 | 38 |
| 3 | 14 | 8.3 | PA plasty, aortopexy | Aortic extension+PA plasty | 44 | 34 |
| 4 | 28 | 10 | TCPC, AoV plasty | Aortic extension+anastomosis of IVC side | 81 | 34 |
| 5 | 17 | 7.1 | Rastelli,PA plasty | Aortic extension+PA plasty, VSD closure | 112 | 32 |
| 18.2±7.6 | 8.4±0.9 | | | 64.8±28.6 | 53.8±38.3 |
| AoV: aortic valve, TCPC: total cavopulmonary connection |
In the four patients (patients 1–4) with a functional single ventricle, the TCPC-type Fontan surgery was performed. The fifth patient underwent biventricular repair using the Rastelli procedure. All patients were followed up on an outpatient basis, with no long-term observations of stenosis or repeat dilatation in any patient. The mean postoperative follow-up period was 53.8±38.3 (range, 32–130) months.
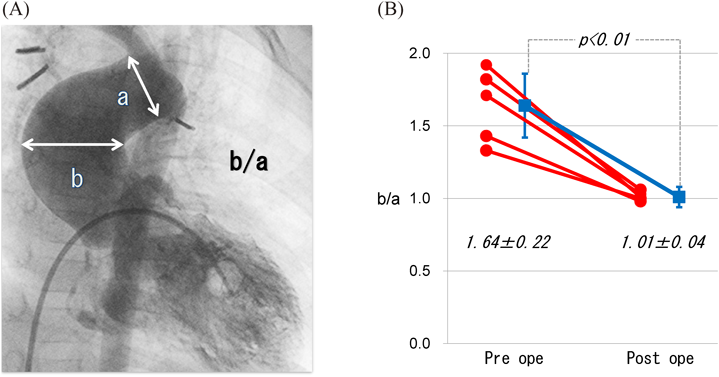
The ratio of the maximum to peripheral diameter of the ascending aorta was 1.64±0.22 preoperatively and was significantly reduced to 1.01±0.36 postoperatively. In all patients, reduction of the ascending aorta diameter and enlargement of the retroaortic space were achieved (Fig. 2A, B).
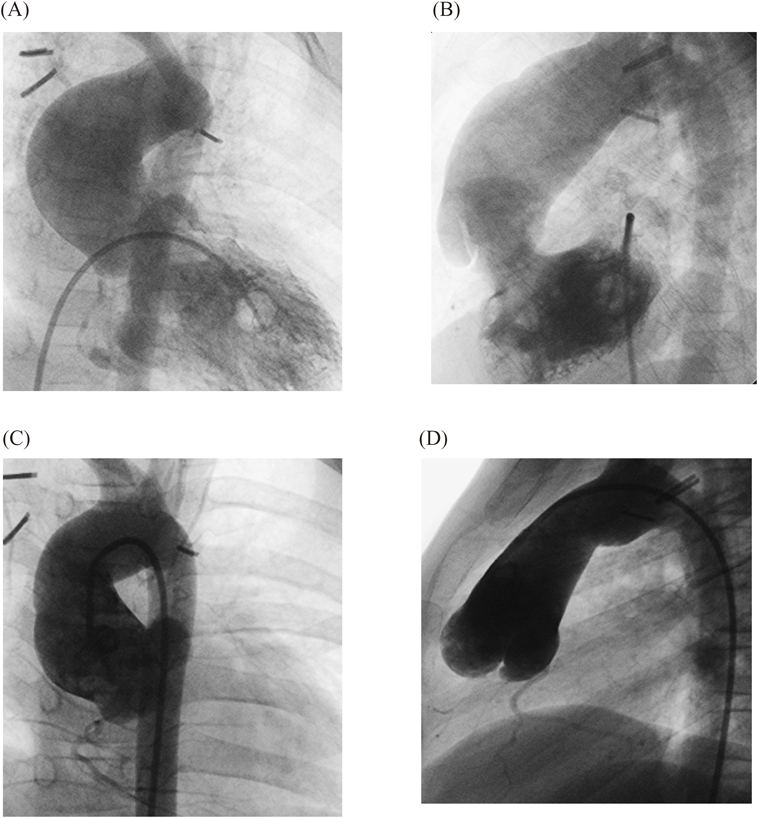
Images of pre- to postoperative changes of two typical cases are shown. Fig. 3 presents lateral view images of patient 1 obtained using aortic angiography preoperatively and 6 years postoperatively. The ascending aorta, which was dilated preoperatively, reduced in size postoperatively, and the retroaortic space enlarged and its size maintained.
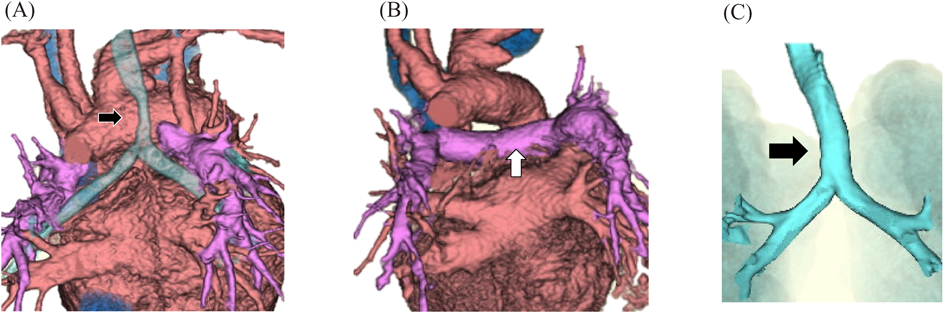
Fig. 4 presents images of patient 3 obtained using contrast-enhanced computed tomography (CT) preoperatively and at 5 months postoperatively. Preoperatively, the central pulmonary artery was absent in the space between the left and right hilar regions, and the trachea was displaced by the aortic arch. However, postoperatively, the central pulmonary artery conduit was interpositioned beneath the aortic arch, and the tracheal stenosis improved.
Compared with healthy individuals, in patients with cyanotic heart disease with stenosis or atresia of the main pulmonary artery (e.g., tetralogy of Fallot, a common right-to-left shunt), pulmonary artery blood flow is decreased and aortic blood flow is increased from the fetal stage. This causes dilatation of the ascending aorta and hypoplasia of the central pulmonary artery. In patients with atresia of the main pulmonary artery, this tendency is exacerbated.1, 2) Therefore, the retroaortic space narrows congenitally.
While performing radical surgery, the minimum diameter of the central pulmonary artery must be maintained. Depending on the patient, the central pulmonary artery can be entirely absent, thereby raising the challenge of how to ensure space for pulmonary artery reconstruction.4)
In addition, enlargement of the ascending aorta and aortic arch can displace the trachea and the left and right bronchi. In some cases, patients may present with symptoms of upper respiratory tract stenosis that complicate perioperative management.1, 2, 4)
As a surgical means of overcoming these two morphological problems (aortic hyperdilatation and narrowing of the retroaortic space), we previously reported the usefulness of aortic extension using the autologous vascular wall.3)
To date, several studies on surgical procedures aimed to eliminate pressure via the aorta. Among these procedures, commonly used procedures are lifting the ascending aorta toward the posterior surface of the sternum1, 2) and posterior traction of the descending aorta.5) Although both procedures are simple and do not require the use of extracorporeal circulation, their effectiveness is limited secondary to an inability to enlarge the retroaortic space. Furthermore, if the ascending aorta is lifted in patients who require multiple surgeries, caution is required regarding the risk for aortic injury when performing resternotomy.1, 4) Moreover, posterior traction of the descending aorta has shortcomings because it requires lateral thoracotomy, which is difficult to simultaneously perform with routine cardiac surgery.
Ascending aorta plication2, 6) is also a relatively easy procedure that does not require extracorporeal circulation and can reduce enlarged aortic diameter. However, performing plication of the lesser curvature side is technically difficult. In addition, when performing plication of the greater curvature side, which is easier to suture, limited effects are realized unless performed in conjunction with a technique for lifting the aorta. Furthermore, the use of long suture lines longitudinally along the aorta can inhibit aortic growth in infants. This raises concerns regarding future re-narrowing of the retroaortic space.
In adults, the usefulness of wrapping the aorta with a vascular graft to reduce the aortic diameter has been reported.7, 8) However, performing this technique for infants is difficult because of problems with growth and challenges associated with installing the vascular graft below the narrow aortic arch.
In contrast, one method for forcibly expanding compressed left and right pulmonary arteries is stent placement within the pulmonary artery.4, 9) Moore et al.9) placed pulmonary artery stents in eight children (9.1–20.0 kg) with left pulmonary artery stenosis and reported that expansion was successfully achieved without any complications. However, growth cannot be expected, and erosion of the aorta may also occur.4) Similarly, compression of the trachea and bronchi can also be treated by internal2, 10) and external stent placement.11) However, for small children, internal stent placement can cause aortobronchial fistulas, airway inflammation, and permanent injury.2, 10) Therefore, safety concerns associated with this procedure still exist. External stent placement11) is effective depending on the patient; however, in patients with little space under the aortic arch, performing the procedure is technically difficult.
Ascending aortic extension that uses a vascular graft to radically expand the retroaortic space has been reported.4, 12) Baker et al.4) performed aortic extension using polytetrafluoroethylene vascular grafts to treat seven children (aged from 7 months to 7 years) with pulmonary artery stenosis and bronchial stenosis after aortic arch reconstruction using methods such as the Damus–Kaye–Stansel procedure and the Norwood procedure. They reported that the procedure could be performed even when lifting the aorta was morphologically difficult. The diameter of the vascular graft used was 16 mm in five cases. Although the diameter of the vascular graft was smaller than that in a healthy adult, no significant pressure difference was expected because of the short distance. However, this approach remains problematic because growth cannot be anticipated, possibly contributing to left ventricular afterload for several subsequent decades.
Another aortic arch extension using the autologous pulmonary artery wall13) has been reported and appears to be an excellent procedure with the possibility of growth in the autologous vascular wall. However, the procedure requires the autologous main pulmonary artery to be thick; thus, if the main pulmonary artery is congenitally absent or small, the procedure cannot be performed. Furthermore, extending the aortic arch portion requires the use of circulatory arrest, and long-term problems such as calcification, stenosis, and dilatation can also arise.13)
Although the present procedure using autologous aorta alone requires extracorporeal circulation and must be performed under cardiac arrest, it enables simultaneous reduction of the aortic diameter and aortic extension. It can also help in preventing the formation of aortic aneurysms and in sufficiently enlarging the retroaortic space. In fact, three of the four patients with a functional single ventricle had no central pulmonary artery, and the left and right pulmonary arteries were nonconfluent. Despite this, after aortic extension, we could create a central pulmonary artery with adequate thickness, and in all four patients, good Fontan circulation was maintained. Furthermore, in patient 3, who had tracheal stenosis, we eliminated the tracheal stenosis without using an internal or external stent. Moreover, because an artificial object is not used, we can theoretically perform this procedure for children of small physique, and we believe that the procedure is excellent because growth is expected.
With regard to the indications for the present procedure, we believe that the procedure is suitable for patients for whom angioplasty with expansion of the central pulmonary artery diameter is difficult because of the enlargement of the ascending aorta and a narrow retroaortic space. Furthermore, the procedure is suitable for patients in whom the aorta causes displacement and stenosis of the trachea and/or bronchi. Requirements include the need for transection of the aorta between the sinotubulat (ST) junction and the site of aortic clamping and suturing of both ends. The aortic clamping forceps need to be prevented from slipping or coming free during the procedure; consequently, an adequate distance should be maintained between the ST junction and the site of aortic clamping to safely cut and saw. The larger the aortic diameter, the greater the extension effect. In contrast, the thinner the diameter, the weaker the effects. Given this, we believe that indications for this procedure should be determined according to the degree of retroaortic expansion that is required. Although extension is more effective when the reduction of the diameter is greater, after angioplasty, the minimum diameter of the ascending aorta must be at least the length of the normal aortic annulus diameter. However, in all five patients, the actual aortic annulus diameter of each patient was larger than normal and smaller than that of the dilated ascending aorta. Therefore, reconstruction was performed so that the diameter was the same as that of the aortic annulus of each patient.