The patient first presented prenatally, at 32 weeks of gestation, to our center for evaluation of a TOF with MAPCAs and a right aortic arch. The baby girl was born at 39 weeks of gestation, by vaginal delivery, with a birthweight of 2,363 g. Her O2 saturation, on room air, was 95% at birth.
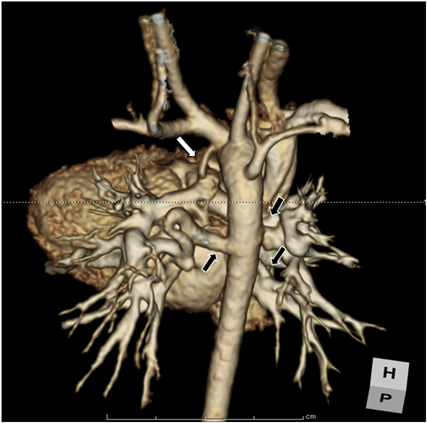
The echocardiography performed at birth confirmed the TOF, including a severely hypoplastic pulmonary valve (annular size, 3.7 mm; Z score, −5.8) and MAPCAs. The branch of the PA was also hypoplastic, with a Nakata index of 76 mm2/m2 and a negative jet from the MAPCAs to the native PA observed in the distal portion. The outlet septum was relatively small and did not show prominent RVOT obstruction. Contrast enhanced computed tomography (CT) imaging was performed on post-natal day 29 to assess the MAPCAs (Fig. 1). Three MAPCAs were identified, with an additional small vessel which we considered to be a remnant of the patent ductus arteriosus (PDA).
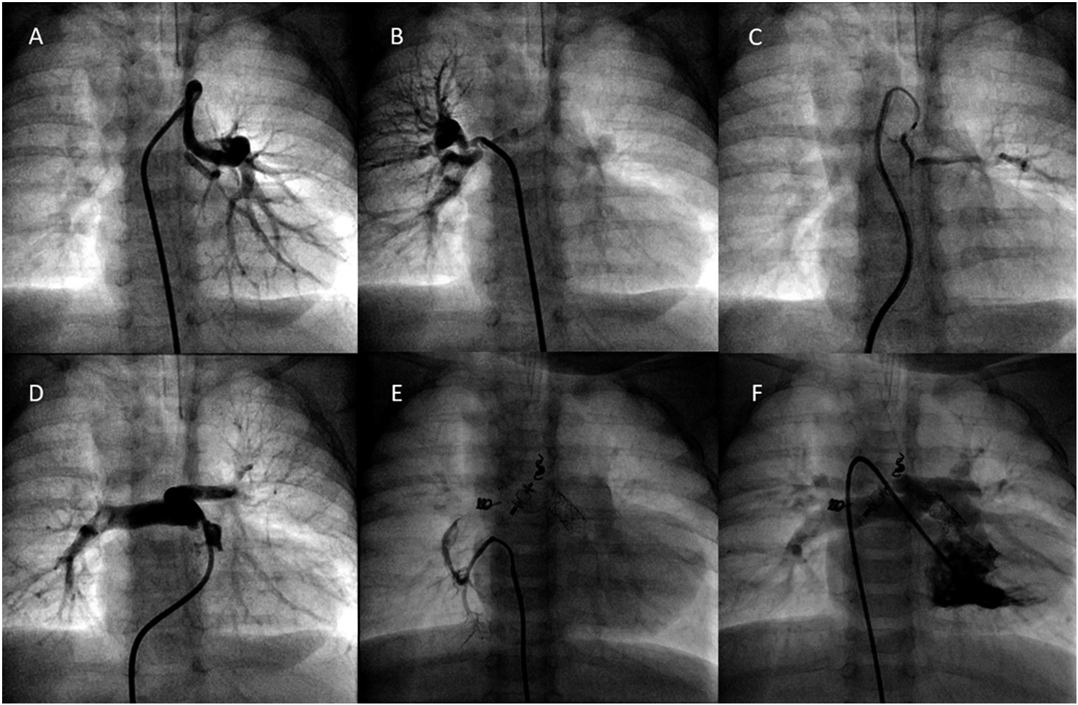
As the baby gained weight, her O2 saturation decreased below 80%, due to inadequate growth of the pulmonary valve and progression of sub-pulmonary stenosis. We performed a catheterization on post-natal day 61 (weight, 3,900 g) to evaluate the MAPCAs directly and for intervention to increase pulmonary blood flow. The pulmonary annular size measured 3.5 mm (Z score, −7.4) with echocardiography then. We identified two large MAPCAs (MAPCA 1 and 2, Fig. 2) on angiogram, one each for the right and left lungs, with both having a dual blood supply to the native PA, which was hypoplastic (Fig. 2). The small vessel thought to be a remnant of the PDA on CT imaging proved to be a small MAPCA (MAPCA 3, Fig. 2), which also perfused the left lung. The Nakata index for the native PA was 154 mm2/m2, and a negative jet was again observed. We proceeded with catheterization to promote antegrade PA blood flow and, hopefully, to enable the occlusion of all the MAPCAs. With a pulmonary annular size of 4.0 mm measured on angiography, we proceeded with balloon pulmonary valvuloplasty (BVP), using a Sterling™ 5×20 mm balloon catheter (Boston Scientific Corporation, USA). After BVP, her O2 saturation increased to 85% on room air. We, therefore, proceeded with occlusion of the MAPCAs 2 and 3, using an AMPLAZER™ vascular plug-II (AGA Medical Corporation, USA) and an ORBIT GALAXY® device (Codman & Shurtleff, Inc. USA), respectively. We then proceeded with occlusion of MAPCA 1, using a Berman angiography catheter, which caused a drop in her O2 saturation to 72% on room air. An increase in the antegrade PA blood flow was needed to allow occlusion of all the MAPCAs and to promote the growth of the native PA. Thus, we decided to perform RVOT stenting, using an Express™ Vascular SD, 6×14 mm, stent (Boston Scientific Corporation, USA). The stent was successfully placed across the pulmonary valve, as the outlet septum was relatively short. O2 saturation increased immediately to 93%, even after occlusion of MAPCA 1, using a Flipper® PDA Closure Detachable Coil (Cook Medical Incorporated, USA). Since stents for RVOT is an off-label use in Japan, we discussed the possible risks of this procedure carefully before the session with the parents, and acquired written consent form. The official approval of institutional review board could not be in time for the procedure, yet it was approved immediately.
Postoperatively, the patient was treated with diuretics to prevent reperfusion injury of the lungs. The patient was able to feed well and gained weight gradually. At 4 months of age, she reached a weight of 5.4 kg, with an O2 saturation of around 90% on room air. Catheter evaluation indicated an increase in the Nakata index to 352 mm2/m2, with a mean PA pressure of 13 to 17 mmHg. A remaining MAPCA was identified, with some residual flow observed in the previously occluded MAPCAs. We proceeded with occlusion of the new MAPCA, using an AZUR® CX18 and ED COIL (KANEKA MEDICAL PRODUCTS, Japan). At 6 months of age, the patient underwent surgical repair using a transannular patch and the RVOT stent was removed completely, without any difficulty. Her post-operative clinical course was good, and she is being followed on an outpatient basis, remaining in a fairly stable state.
Considering the high risk of complete TOF repair and palliation using the modified BTS in the neonatal and early infancy period,1–5) RVOT stenting has emerged as a viable alternative palliative option for cyanotic TOF, particularly for infants with severe comorbidities, which might be negatively impacted by a modified BTS procedure and low birth.3) With accumulating experience, RVOT stenting has also become the preferred option for the treatment of patients with a hypoplastic PA, defined by a Z-score <−2.6) RVOT stenting has also been used for the treatment of pulmonary atresia associated with perforation of the pulmonary valve.7) RVOT stenting is now widely used as a palliative option for patients with a TOF type physiology.
The management strategy for TOF with MAPCAs is more complex compared to TOF without MAPCAs. Some centers perform neonatal PA rehabilitation, using shunting to promote the PA growth without translocation of the MAPCAs,8) while other centers opt for single stage UF, which may lead to better long-term outcomes.9) Regardless of the strategy used, the presence of a native confluent PA is a very important factor to consider. In our case of TOF with MAPCAs, all MAPCAs had a dual blood supply to the confluent native PAs. In such a case, UF may not be necessary, but augmentation of the native PA is often required, prior to intra-cardiac repair, with creation of an aortopulmonary window (APW) being an option for this strategy.10) In our case, as the angiogram confirmed that all MAPCAs are connected to the confluent native PAs with a dual supply, we performed a palliative intervention to promote antegrade blood flow in the native PA. Although having dual supply MAPCAs is not necessarily the same as an adequate connection that does not require unifocalization, we assumed that our patient had enough pulmonary vasculature for intra-cardiac repair without unifocalization from the angiogram and the CT performed prior to catheter, if we could successfully promote antegrade pulmonary blood flow. Initial BVP was effective, with the O2 saturation increasing from 78% to 85%, but was ineffective in increasing the antegrade blood flow sufficiently in the native PA to occlude all MAPCAs. Since there was a prominent negative jet from the MAPCAs to the native PA, we thought it necessary to occlude all the MAPCAs to promote antegrade pulmonary blood flow to promote native PA growth. Occluding the MAPCAs later in another session was an alternate option, thought we thought RVOT stenting may facilitate antegrade blood sufficiently to occlude the MAPCAs in one session, reducing the number of catheter and total radiation exposure as well. This is why we performed RVOT stenting, which was successful in increasing the antegrade PA blood flow sufficiently to occlude all MAPCAs, as well as to increase O2 saturation. RVOT stenting is less invasive and provides a higher diastolic pressure compared to BTS or APW, which is preferable with respect to coronary perfusion, and may provide a more stable inter-stage status. As for the selection of the stent profile, we chose the size according to the report from Toronto group,4) using a stent which is 1 to 2 mm larger compared to the RVOT diameter in diastole. For we have already performed BVP with a 5 mm balloon, we chose a 6 mm stent for stability. As the surgeons had already concluded that there was no way to preserve the native pulmonary valve for it was too small, we decided to place the stent across the pulmonary valve to reduce the risk of stent migration. Since RVOT stenting in a patient with a small outlet septum may carry a risk of impinging the aortic valve and of aortic regurgitation,11) it is preferable to place the stent at the level of pulmonary valve to avoid lower positioning of the stent as in the present case. Thus, we successfully placed the stent without any complications. The Nakata index increased dramatically after stenting, from 154 mm2/m2 to 352 mm2/m2 at 2 months after the intervention, allowing the patient to undergo intra-cardiac repair at 6 months of age. RVOT stenting causes free pulmonary regurgitation hemodynamically, yet it created sufficient antegrade pulmonary blood flow to promote pulmonary artery vasculature growth as described in previous report.6) Although a previous study indicated that RVOT stents cannot be removed completely in most cases,12) we were able to remove the stent successfully. Reports with higher removal rate seems to have a relatively shorter palliation period.13) The successful removal of stent may have been due to relatively short duration of palliation (4 months). Moreover, our surgical management with RVOT stenting was successful in promoting sufficient PA growth for intra-cardiac repair using only one open-heart surgery. Since patients with TOF and MAPCAs will require repeat RVOT repair, it is important to consider a strategy to reduce the number of initial procedures required. And in this context, evading unifocalization is preferable for Mainwaring RD et al. reported that 18% of patients who underwent unifocalization required revision of the distal unifocalized bed and that most of these patients had a single-stage repair.14)