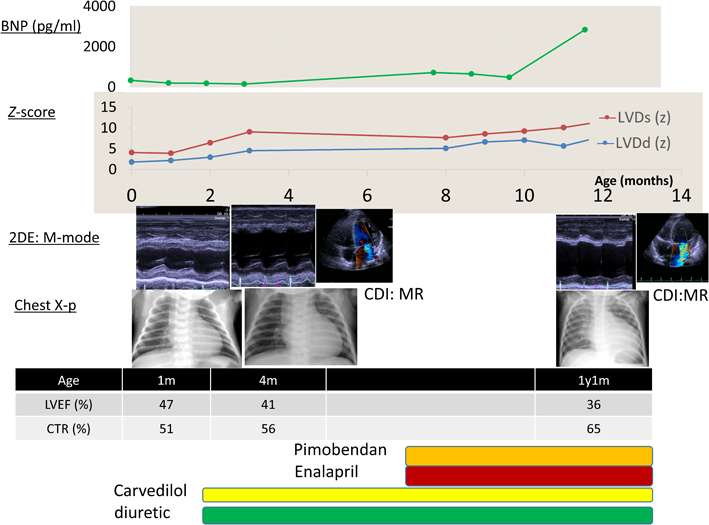
At 14 days of age, the infant presented with cyanosis while crying and was referred to our institution at 1 month of age. The patient was born to a 27-year-old healthy mother at term (40 weeks of gestation) and had a birth weight of 3,010 g. On physical examination, his weight was 3,200 g, which indicated poor weight gain, a likely reason for his poor sucking effort. His heart rate was measured at 120 bpm. Physical findings were significant for a II/VI systolic pansystolic murmur, with third sound and a 1-cm liver edge palpable on the right side. Initial blood evaluation revealed an elevated B-type natriuretic peptide (BNP) level (331.7 pg/mL). Electrocardiography revealed high R voltage from V4 to V6 and confirmed the LV hypertrophy. Two-dimensional (2D) echocardiography revealed the two-layer structure of the noncompacted (N) and compacted (C) layers and an N/C ratio of >2.0 at the lateral wall and apex (Fig. 1 (A1)). The Doppler echocardiography revealed that the flow within the deep intertrabecular recesses was in continuity with the LV cavity (Fig.1 (A2)). Moderate mitral valve regurgitation was also observed.
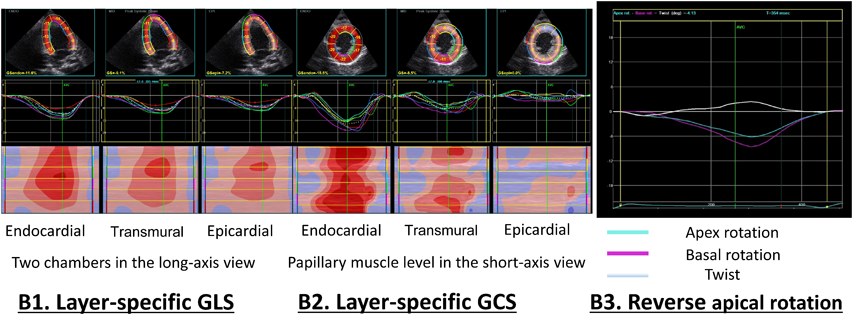
At 2 months of age, the patient’s cardiac function was reevaluated by standard procedures recommended by the American Society of Echocardiography5) and the z-score (http://zscore.chboston.org/Home/About; Table 1). In the evaluation with the M-mode electrocardiography, the z-scores of LV systolic dimension (LVDs), LV diastolic dimension (LVDd), LV posterior wall thickness at diastole (LVPW), compacted layer in LVPW, and LV ejection fraction (LVEF) by 2D biplane Simpson were 6.4, 3.0, 4.0, −3.5, and 54.3%, respectively. We performed 2D speckle tracking (2DST) echocardiography of a myocardial layer-specific strain. 2DST analyses were retrospectively performed using a vendor-specific software (EchoPAC PC version 201, GE Healthcare). Manual tracings of the endocardial border during end-systole in 3 apical views (Fig. 2 (B1), and layer-specific 2DST of 2 chambers in the long-axis view) and at 3 levels of the short-axis views (Fig. 2 (B2), the layer-specific 2DST of the papillary muscle levels to short-axis view) were performed to measure the global longitudinal strain (GLS) and global circumferential strain (GCS). The software determined strain values at transmural (not mid-wall) and endocardial locations, epicardial strain, and the ratio of endocardial longitudinal (circumferential) strain at end-systole (GL(C)S) to epicardial GL(C)S. The patients endocardial, transmural, and epicardial GLS at 2 chambers were −11.6%, −9.1%, and −7.2%, respectively, while his endocardial, transmural, and epicardial GCS values at the middle levels were −15.7%, −8.8%, and −4.6%, respectively. We administered diuretic medications (furosemide 3 mg/kg/day and spironolactone 1 mg/kg/day) and a beta-blocker (carvedilol: initial dose, 0.05 mg/kg/day; target dose, 0.2 mg/kg/day) for HF. Cardiac magnetic resonance imaging performed at 3 months of age confirmed the characteristic features of numerous trabeculations and deep intertrabecular recesses at the apex of the LV. No delayed enhancement was observed. The LV volume was examined with cardiac magnetic resonance imaging using the z-score (http://www.parameterz.com/cmr/jcmr09). The Left ventricular end diastolic volume (LVEDV) was 16.6 mL with a z-score of 1.47, the Left ventricular stroke volume (LVESV) was 8.4 mL with a z-score of 2.75, and LVEF was 49.5%. The cardiac catheterization findings at 4 months of age demonstrated enlargement of the LV and right ventricles. No evidence of pulmonary hypertension was found, and the wedge pressure was approximately 9 mmHg. In addition, at 4 months of age, the patient developed a urinary tract infection, which improved with antibiotic treatment. The minimum neutrophil count was 168 of the white blood cell count, 3% of total white blood cell, when he was 5 months old. We performed a genetic analysis and confirmed the presence of the TAZ variant c.461-2A>C in both the infant and the mother when he was 5 months old. The variant is expected to result in the loss of TAZ function but is extremely rare in the population and in silico analysis predicts as pathogenic (Table 2). The mother showed no clinical evidence of cardiomyopathy.
Table 1 Serial change in cardiac functions | | | 2 months | 9 months |
|---|
| | Length (cm) | 53.2 | 68.5 |
| | Weight (kg) | 5.2 | 7.8 |
| M-mode |
| | LVDs (z-score) | 6.4 | 8.6 |
| | LVDd (z-score) | 3.0 | 6.6 |
| | LVPW (z-score) | 4.0 | 15.3 |
| | LVPWC (z-score) | −3.5 | −2.7 |
| Other parameters |
| | LVESV (z-score) | 4.4 | 6.8 |
| | LVEDV (z-score) | 3.6 | 4.2 |
| | LVEF (%) | 54.3 | 28.4 |
| | LV E/é | 11.3 | 14.9 |
| | N/C ratio | 2.1 | 2.5 |
| Myocardial layer-specific strain using 2DST |
| GLS | Global (%) | Endocardial GLS | −9.0 | −9.7 |
| | Transmural GLS | −7.2 | −7.7 |
| | Epicardial GLS | −5.9 | −6.3 |
| | Endo /Epi ratio | 1.5 | 1.5 |
| GCS | Global (%) | | | |
| Base (%) | Endocardial GCS | −9.2 | −7.3 |
| | Transmural GCS | −5.0 | −4.7 |
| | Epicardial GCS | −3.4 | −3.2 |
| | Endo /Epi ratio | 2.7 | 2.3 |
| Middle (%) | Endocardial GCS | −15.7 | −8.1 |
| | Transmural GCS | −8.8 | −4.6 |
| | Epicardial GCS) | −4.6 | −3.0 |
| | Endo /Epi ratio | 3.4 | 2.7 |
| Apex (%) | Endocardial GCS | −18.5 | −8.5 |
| | Transmural GCS | −8.5 | −3.9 |
| | Epicardial GCS) | −3.2 | −3.2 |
| | Endo /Epi ratio | 5.8 | 2.7 |
| Abbreviations: LVDd; left ventricular diastolic dimension, LVDs; left ventricular systolic dimension, LVPW; left ventricular posterior wall thickness at diastole, LVPWC; compacted layer in left ventricular posterior wall at diastole, LVESV; left ventricular systolic volume, LVEDV; left ventricular diastolic volume, LVEF; left ventricular ejection fraction by 2D Biplane Simpson, LV E/é; left ventricular inflow E wave/ tissue Doppler Imaging of lateral mitral annulus, é wave, N/C ratio; ratio of noncompacted layer to compacted layer, 2DST; two-dimensional speckle tracking echocardiography, GLS; global longitudinal strain, GCS; global circumferential strain. |
Table 2 Variant identified in the patient| cDNA change | Protein change | db SNP | gnomAD (All Individuals) (%) | gnomAD (East Asian) (%) | HGVD (%) | CADD |
|---|
| c.461-2A>C | | — | — | — | — | 23.2 |
gnomAD, https://gnomad.broadinstitute.org
HGVD databases, http://www.hgvd.genome.med.kyoto-u.ac.jp
CADD; https://cadd.gs.washington.edu |
The infant’s clinical course is shown in Table 1 and Fig. 3. We initially treated the patient with diuretics and carvedilol, which alleviated the HF. However, over time, the HF progressed and could not be adequately controlled. An ACE inhibitor (enalapril: initial dose, 0.05 mg/kg/day; target dose, 0.2 mg/kg/day) and calcium sensitizer (pimobendan, 0.03 mg/kg/day) were added to his regimen. By 8 months of age, the patient’s condition had stabilized, and he exhibited normal cognitive development. However, by 9 months of age, his cardiothoracic ratio gradually increased; his LVDs, LVDd, LVESV, LVEDV, and LV E/e had increased; his LVEF had decreased; and mitral valve regurgitation gradually developed. In the 2DST echocardiography analysis, his endocardial, transmural, and epicardial GCS at the middle levels were decreased to −8.1%, −4.6%, and −3.0, respectively, as compared with the data at 2 months of age. The HF showed clinical signs of progression. Moreover, retrospective analysis of cardiac rotation revealed normal heart rotation, counterclockwise rotation of the cardiac base, and clockwise rotation of the cardiac apex. His cardiac rotation indicated that the cardiac base and apex had rotated in the counterclockwise direction, the so-called reverse apical rotation (RAR), with abnormal rotation of the cardiac apex in the same direction (Fig. 2 (B1), (B3)). At 12 months of age, the infant died due to progressive HF induced by dilated cardiomyopathy (DCM) with LVNC. At postmortem, he was diagnosed with BTHS with LVNC and neutropenia. However, we could not confirm the growth delay and skeletal myopathy and did not examine urinary excretion for 3-methylglutaconic acid. Informed consent was obtained as inclusion agreements based on the “Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.”
Allelic variants of BTHS were speculated in our case because of the confirmed LVNC and neutropenia. Our case was consistent with a DCM phenotype of LVNC due to TAZ variants, and the patient developed HF with a poor prognosis. BTHS with LVNC often responds well to conventional medical therapy, and the prognosis of most patients is good after 5 years of age in cases where cardiac function recovers during infancy.6) Our patient may have received delayed medical therapy with diuretic and beta-blocker for HF, administered at 2 months of age. If early onset of HF with BTHS is predicted, treatment using multiple medications should be initiated as early as possible. However, in a recent study, patients with the DCM phenotype of LVNC due to TAZ variants had higher mortality rates and more severe cardiac events than those with other phenotypes, with no significant difference in mortality between the TAZ-positive and other DCM phenotype groups.7) Several lines of evidence support the pathogenic role of the novel splicing variant. First, variants were found to segregate with the disease in the patient; the mother was carrier of the TAZ variant. Second, the frame-shift variant was neither present in gnom AD nor HGVD databases (https://gnomad.broadinstitute.org and http://www.hgvd.genome.med.kyoto-u.ac.jp). LVNC patients with TAZ variants have a higher frequency of early onset of disease and poor prognosis. Identification of TAZ variants suggests possible clinical advantages and tailored therapeutic interventions may become available. Therefore, identifying patients who should be observed with greater caution during the early stage of the disease remains a critical issue.
LVNC is a recently defined cardiomyopathy characterized by a pattern of prominent trabecular meshwork and deep intertrabecular recesses.8) Prognosis and clinical features vary between asymptomatic and symptomatic patients and have the potential for progressive myocardial dysfunction. Complications of LVNC include HF, thromboembolic events, arrhythmias, and sudden cardiac death.9) Recently, increasing attention has been focused on the role of 2DST echocardiography in the assessment of patients with LVNC as it may aid in early detection, development of more specific diagnostic criteria, or serial monitoring of patients with subclinical ventricular dysfunction. Studies have demonstrated that strain imaging and strain rate imaging can reveal subclinical impairment in patients with LVNC with preserved ejection fractions and decreased deformation near the apical segments.10) In a recent report,11) among patients with LVNC with preserved LVEF (50%) and without LV dilation (LVEDV z-score <2), the GLS was decreased. The GLS and GCS are decreased in pediatric patients with LVNC in comparison with control subjects. Moreover, among patients with LVNC those with adverse outcomes, poor prognosis, or who required heart transplantation had lower 2DST echocardiography, including GLS and GCS, than patients with benign outcomes and a good clinical course or without the need for heart transplantation. Recently, the proposed new 2D strain software has shown the potential to assess layer-specific strain and introduced the speckle-tracking echocardiogray technology, which allows noninvasive bedside assessment of layer-specific myocardial deformations.12) This new, sensitive indicator is highly effective in detecting cardiac dysfunction in various cardiac diseases, including those in infants.13) In our case, the infant had preserved LVEF and GCS, especially endocardial GCS; however, LV dilatation and the GLS at all layers were decreased. The potential causes of the transmural strain gradient include transmural differences in wall stress, characterized by increases in end-diastolic wall stress toward the endocardium. Therefore, the endocardial fibers are stretched longer than the epicardial fibers during end-diastole, which results in increased fiber shortening in the endocardial layer during systole. An additional explanation for the transmural strain gradient could be differences in coronary perfusion and metabolism between the endocardial and epicardial layers, higher metabolic rates, greater oxygen extraction, and greater coronary flow in the endocardium than in the epicardium.14)
An additional analysis was performed using 2DST echocardiography to determine the rotation mechanism of the LV. The normal twisting motion of the LV during ejection consists of clockwise rotation of the base and counterclockwise rotation of the apex (when viewed from the apex). In some patients with LVNC, the mechanism is characterized by rotation of the apex and base in the same direction, the so-called RAR. Nawaytou et al. reported on the LV rotational mechanism in children with LVNC.15) They stated that RAR occurs in 39% of children with LVNC which may have a prognostic rather than a diagnostic value. They speculated that the causes of RAR are disarray in the midmyocardial fibers and concluded that premature untwisting of the LV during ejection might be an even more worrisome indicator of LV dysfunction. In our case, the patient manifested RAR when he was 9 months old (Fig.2 (B3)). An autopsy was not performed on our patient; hence, we were unable to determine the mechanism underlying the relationship between myocardial coronary flow in the endocardium and the preserved endocardial GCS and even decreased LVEF and GLS; however, we assume that there is a relationship between the directions of the myocardial fibers and RAR and LVNC patients with TAZ variants. Future prospective studies with large patient populations are needed.
In conclusion, this case demonstrates the impact of the DCM phenotype of LVNC and emphasizes the cardiac functions using 2DST echocardiography in LVNC patients with TAZ variants. TAZ gene analysis is effective for BTHS diagnosis, and 2DST echocardiography analysis might be an effective prognosis tool for the DCM phenotype of LVNC with TAZ mutation. Most patients are diagnosed in the first 4 months of life. TAZ-positive patients have significantly earlier disease onset than those with other DCM phenotypes of LVNC. Our case suggests the impact of the DCM phenotype of LVNC.