Kawasaki disease (KD) is an acute vasculitis of unknown origin, and causes coronary artery abnormalities such as giant coronary aneurysms and dilatation. Treatment of acute KD needs high-dose intravenous immunoglobulin (IVIg) therapy in about 94.6% of patients.1) About 20% of these patients fail to respond to the first IVIg therapy, of whom about half respond to a second IVIg therapy. However, some patients fail to respond to additional IVIg, and coronary artery abnormalities are likely to arise in such patients. Additional treatments for IVIg-resistant KD have been discussed, including steroids, infliximab, immunosuppressant, and plasmapheresis. No therapies have yet been identified as effective for cases of intravenous IVIg-resistant KD. Hamada et al. reported that primary therapy combining IVIg and ciclosporin was safe and effective for achieving favorable coronary artery outcomes in KD patients predicted to prove unresponsive to IVIg.2) We report a case in which administration of infliximab was successful for a child with KD refractory to additional therapy with IVIg and oral ciclosporin.
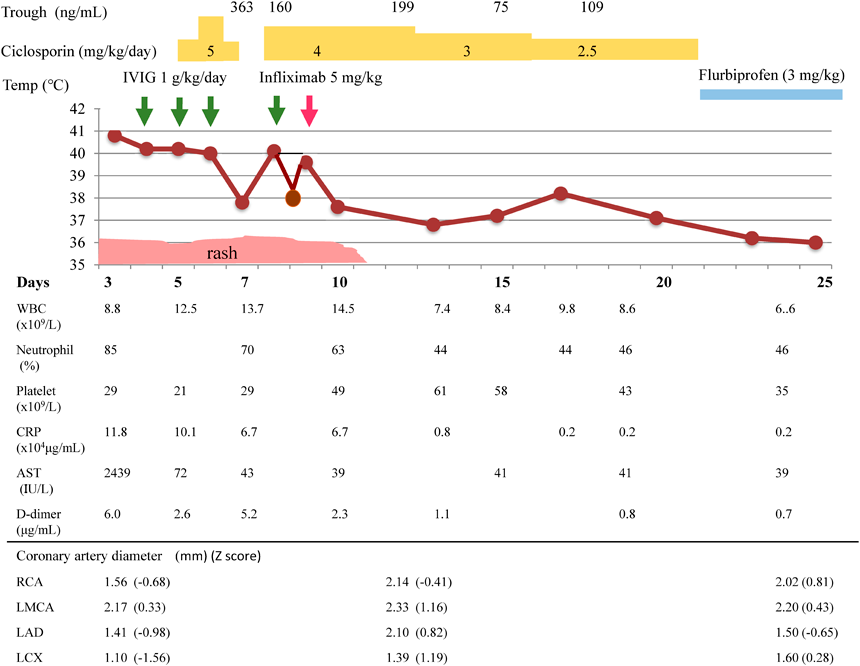
A 2-year 6-month-old boy (body weight, 13.4 kg) developed a fever of 39.0°C and redness at the Bacille Calmette-Guérinon inoculation site in the morning 2 days before admission. The next day, he showed erythema of the back and redness of the lips. He was then transferred to our hospital on day 3 of illness, because Kawasaki disease was suspected. On admission, body temperature was 40.8°C, and he appeared ill. The patient showed left cervical lymphadenopathy and swelling of the palms and soles. After admission, the eyes developed bilateral conjunctival injection. The patient was then seen to show 5 principle symptoms of KD (Fig. 1). Quick tests for Streptococcus pyogenes and adenovirus yielded negative results. Testing for Epstein-Barr virus showed negative results for IgM, and positive results for IgG. Testing for cytomegalovirus likewise showed negative results for IgM and positive results for IgG. On admission, laboratory testing showed: aspartate aminotransferase (AST), 2,439 IU/L; alanine aminotransferase (ALT), 1,142 IU/L; gamma-glutamyl transpeptidase, 280 IU/L; and total bilirubin level, 2.1 mg/dL (Table 1). C-reactive protein (CRP) concentration was 11.8 mg/dL. White blood cell count (WBC) was 8.8×109/L (85% neutrophils), and platelet count was 294×109/L. Fibrinogen and D-dimer levels were 8.13 g/L and 6.0 µg/mL, respectively. Kobayashi, Egami, and Sano scores, each of which predicts IVIg resistance, were 8, 5, and 3, respectively.3–5) Cardiothoracic ratio on chest X-ray was 53%, and the left ventricular end-diastolic dimension and ejection fraction from 2-dimensional echocardiography (2DE) were 34 mm and 74%, respectively. Although tissues surrounding proximal sites at the major coronary arteries on 2DE appeared highly echoic, diameters at the right coronary artery, left main truncus, and left anterior descending artery were 1.56 mm (Z score of −0.68), 2.17 mm (Z score of 0.33), and 1.41 mm (Z score of −0.98), respectively. Twelve-lead electrocardiography revealed tachycardia at a rate of 150 beats/min. IVIg was slowly administered at 1 g/kg body weight/day for about 24 h intravenously for 3 days. On day 6, WBC count and percent neutrophils were 20.7×109/L and 79%, respectively. Ciclosporin was started at a dose of 5 mg/kg/day. Body temperature decreased to 37.2°C by day 7, then increased again to 40.6°C on day 8. IVIg was administered again at 1 g/kg/day, but temperature was 39.3°C on day 9. General condition was poor, and the patient remained asleep in bed almost all day. He could not speak. Chest computed tomography showed no pulmonary abnormalities. Within 2 h of starting administration of intravenous infliximab, fever subsided and his condition improved. Eruptions had entirely resolved by day 10, and condition with bilateral conjunctival injections improved by day 12. No coronary artery lesions were detected on 2DE at any stage. Ciclosporin dose was gradually decreased from day 17, and was stopped on day 23. This combination treatment for severe inflammation with resistance to IVIg proved successful. The patient experienced transient hirsutism as an adverse effect of ciclosporin administration. Although we used ciclosporin and infliximab at the same time, no adverse events involving infections were seen. Aspirin was not used as an anti-inflammatory agent, because of the elevated AST and ALT levels.
Table 1 Laboratory data| CBC |
|---|
| WBC | 8.8×109/L |
| Neut | 84.5% |
| RBC | 4.15×1012/L |
| Hb | 10.5 g/dL |
| Ht | 34.7% |
| Plt | 294×109/L |
| Biochemical |
|---|
| PT | 85% |
| PT-INR | 1.11 |
| APTT | 29 sec |
| Fbg | 8.13 g/L |
| D-dimer | 6 µg/mL |
| TP | 6.5 g/dL |
| Alb | 3.4 g/dL |
| BUN | 11 mg/dL |
| Cre | 0.31 mg/dL |
| UA | 3.4 mg/dL |
| Na | 134 mEq/L |
| K | 4.5 mEq/L |
| Cl | 97 mEq/L |
| Ca | 9.2 mg/dL |
| AST | 2,439 IU/L |
| ALT | 1,142 IU/L |
| CK | 89 IU/L |
| T-Bil | 2.1 mg/dL |
| CRP | 11.76 mg/dL |
| BNP | 24.5 pg/mL |
| IgG | 603 mg/dL |
| IgA | 77 mg/dL |
| IgM | 77 mg/dL |
We presented the case of a 2-year-old boy with Kawasaki disease refractory to combined IVIg and ciclosporin therapy but responded to infliximab therapy. Both ciclosporin and infliximab have been established as the second-line therapy for patients with Kawasaki disease refractory to IVIg who are at high risk for the development of coronary arterial aneurysm; however they modulate the different pathways in pathophysiology of Kawasaki disease. Ciclosporin mainly targets the calcium-nuclear factor of activated T-cells (NFAT) in immune cells and inhibits activated T-cells, whereas infliximab inhibits tumor necrosis factor (TNF)-α which plays a key role in the development of coronary arterial aneurysm.6)
In our present case, combined IVIg and ciclosporin therapy failed to resolve the clinical manifestations associated with Kawasaki disease. The KAICA trial study demonstrated that the incidence of coronary artery abnormalities was significantly lower in subjects with combined IVIg and ciclosporin therapy than in control subjects (2). Combination therapy with IVIg and ciclosporin is likely to be an intensified first-line theapy. In that study, however, there were the greater number of relapsed patients with combined IVIg and ciclosporin therapy (27%), and these patients also received additional therapy, including infliximab therapy in 4 subjects. Otherwise, previous reports have shown that 25.0% patients are refractory to infliximab as the second-line therapy for Kawasaki disease.7) Either ciclosporin or infliximab can be chosen as the second-line therapy for Kawasaki disease refractory to IVIg. Ciclosporin therapy can be continued for 3–4 weeks, whereas infliximab therapy can only be used once for each case of acute KD vasculitis and should be performed by day 10 of illness. Ciclosporin is thus better to used than infliximab during the early course of disease in IVIg-resistant KD patients. Early combination therapy with ciclosporin and infliximab for IVIg-resistant cases appears useful. In the first line therapy, combination therapy with IVIg and steroid such as RAISE study is also one method in patients who are suspected as IVIg resistance.8) However, it is needed more than 5 days for treatment of the RAISE therapy. Therefore, we didn’t use the steroid therapy. The evaluation of the response for the treatment must be done promptly. The other should be chosen as the third-line therapy when the second-line therapy fails. Absolutely, plasmapheresis can be also alternative to these immune modulators. Plasmapheresis started at around 10 days would likely be useful.
In the present case, fever persisted and WBC increased despite decreases in CRP, AST and ALT after the initial IVIg therapy. WBC, especially neutrophil counts, may be a marker of a therapeutic effect of acute KD. Unfortunately, we did not analyze cytokine profiles in the present patient. Although serum levels of inflammatory cytokines including TNF-α, interferon-γ, interleukin (IL)-6, IL-8, IL-17, G-CSF, MCP-1 and soluble IL-2 receptor increases in patients with KD patients. Patients with KD refractory to IVIg therapy should be treated with an appropriate drug and at an optimal timing.9)
Combined therapy using IVIg, ciclosporin and infliximab in that order proved useful against severe acute KD vasculitis. To prevent coronary abnormalities, treatment with optimal additional therapy at an optimal time during the early course of acute KD and rapid strategies are important.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee with the 1964 Helsinki declaration and its later amendments.
Author Contributions
YT drafted the manuscript. ET and KF contributed to the treatment of the patient. ET reviewed the manuscript. IS and KK supervised the manuscript.
All authors have read and approved the final manuscript.
引用文献References
1) Makino N, Nakamura Y, Yashiro M, et al: The nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr Intern 2019; 61: 397–403
2) Hamada H, Suzuki H, Onouchi Y, et al: KAICA trial Investigators: Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): A randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet 2019; 393: 1128–1137
3) Kobayashi T, Inoue Y, Takeuchi K, et al: Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006; 113: 2606–2612
4) Egami K, Muta H, Ishii M, et al: Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 2006; 149: 237–240
5) Sano T, Kurotobi S, Matsuzaki K, et al: Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr 2007; 166: 131–137
6) Weiss JE, Eberhard BA, Chowdhury D, et al: Infliximab as a novel therapy for refractory Kawasaki disease. J Rheumatol 2004; 31: 808–810
7) Masuda H, Kobayashi T, Hachiya A, et al: Committee of Survey on Infliximab use for Kawasaki disease: Infliximab for the treatment of refractory Kawasaki disease: A nationwide survey in Japan. J Pediatr 2018; 195: 115–120
8) Kobayashi T, Saji T, Otani T, et al: RAISE study group investigators: Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): A randomised, open-label, blinded-endpoints trial. Lancet 2012; 379: 1613–1620
9) Hamada H, Suzuki H, Abe J, et al: Inflammatory cytokine profiles during Ciclosporin treatment for immunoglobulin-resistant Kawasaki disease. Cytokine 2012; 60: 681–685