Double outlet left ventricle (DOLV) is a rare conotruncal anomaly with an unusual ventriculoarterial connection where both great arteries arise entirely or predominantly from morphologic left ventricle.1, 2) While bilateral absent coni was considered a diagnostic prerequisite in the past, recent consensus documents describe four different conal configurations, namely bilateral conus, subpulmonary conus, subaortic conus and absent conus.1) Anatomical variations associated with DOLV include presence or absence of ventricular septal defect (VSD), location of the VSD, great artery relationship, presence or absence of pulmonary stenosis, hypoplasia of ventricle and atrioventricular valves.3) As pulmonary stenosis and unfavourable streaming of deoxygenated blood are common, clinical presentation often mimics tetralogy of Fallot or transposition of great arteries.1) Treatment strategies are dependent on multiple factors. Univentricular approach is chosen if a ventricle or atrioventricular valve is small.4, 5) The anatomy of two infants with DOLV are highlighted to provide an algorithm of management with different therapeutic options.
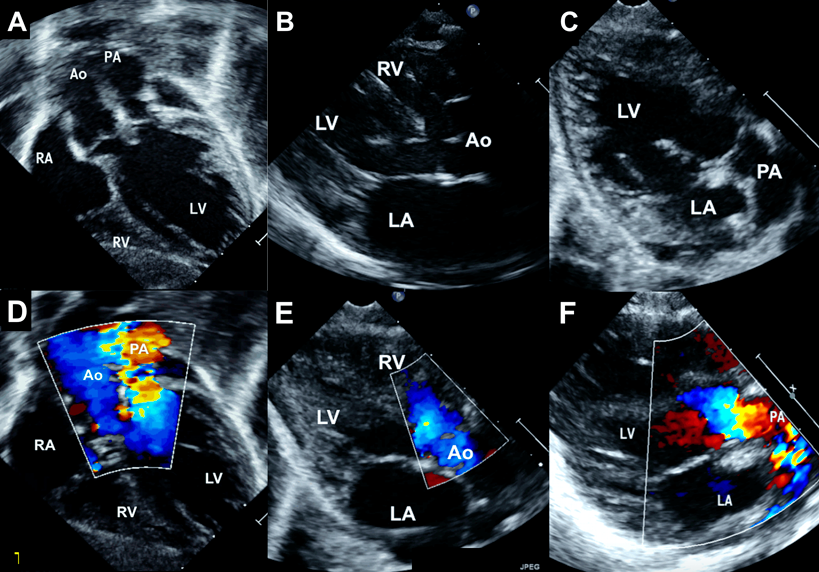
A male infant aged one month weighing 4.1 kg presented with central cyanosis and oxygen saturations of 70%. Echocardiography revealed DOLV where both great arteries were arising entirely from the left ventricle (Fig. 1). A subaortic VSD associated with 50% aortic override streamed right ventricular blood into the ascending aorta. There was no conal tissue and both semilunar valves were in direct fibrous continuity with the mitral valve. The oxygenated blood from the left ventricle streamed into the unobstructed pulmonary artery. The great artery relation was normal and a single coronary artery was seen from the anterior aortic sinus (Fig. 2). During surgery at one month of age, the right ventricle was routed to the right and posterior aortic root by a VSD patch followed by an arterial switch operation, coronary transfer without any Lecompte manoeuvre. At a follow-up of six years, the patient was doing well.
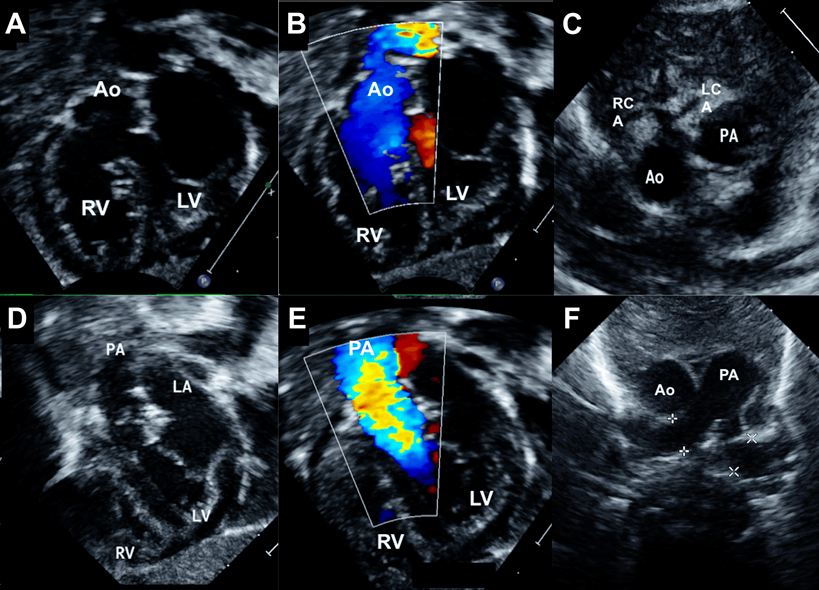
A 2-month-old female infant weighing 2.5 kg with severe cyanosis and oxygen saturation of 58% was diagnosed as DOLV, large subaortic VSD with 30% aortic override, severe annular pulmonary stenosis with peak gradient of 73 mmHg (Fig. 3). Both great arteries were almost completely arising from the left ventricle. The malposed aortic root was left and anterior to the pulmonary artery (Fig. 4). The clinical presentation mimicked transposition of great arteries and pulmonary stenosis with poor inter circulatory mixing. He was planned for staged surgery with initial aortopulmonary shunt to provide additional pulmonary blood flow along with atrial septectomy to improve intercirculatory mixing of blood. The parents declined surgery.
DOLV was described as an anatomical entity fifty years ago, attributed to differential conal absorption in the embryo.2, 6) Presence of a small conal flange separating the semilunar valves from the mitral valve is still accepted in the definition of DOLV, if both great arteries originate from the left ventricle.1, 7) Diagnosis may be made by echocardiogram or angiogram either by conal criteria or by override criteria. A combination of unfavourable streaming of deoxygenated blood often associated with severe pulmonary stenosis leads to early onset and more severe cyanosis, as noted in our patients.
Morphological classifications are similar to double outlet right ventricle (DORV) and based on presence or absence of VSD, relation of VSD to the semilunar valves, presence or absence of pulmonary stenosis, aortic outflow obstruction and associated hypoplasia of right ventricle and tricuspid valve.1) The ventricular septal defect is commonly subaortic in location and seen in three-fourth of patients, as noted in both of our patients. Unlike DORV, remote VSD is extremely rare. Patients with intact ventricular septum are also rarely seen.8) The commonest great artery relationship is d-malposed aorta, seen in half of the patients, even though it was normal in the first patient and l-malposed in the second patient.
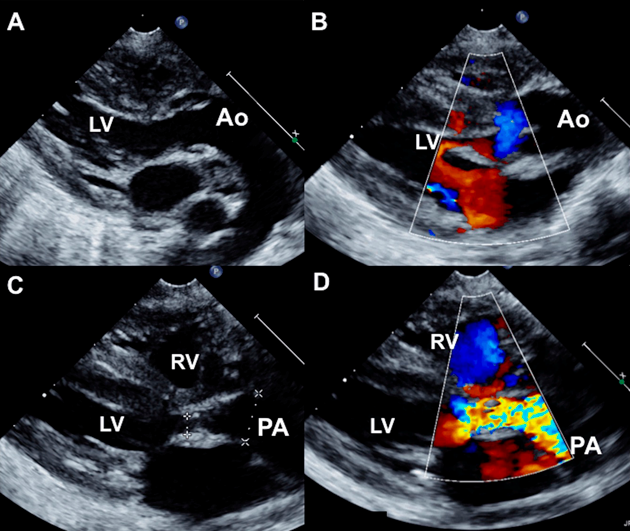
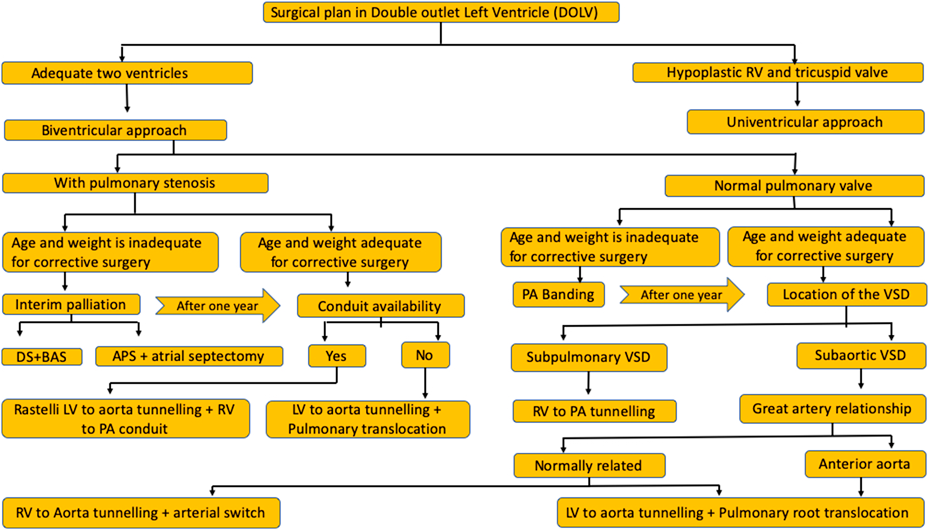
Surgical algorithm varies with presence or absence of pulmonary stenosis. (Fig. 5) DOLV with unobstructed normally functioning pulmonary valve can be dealt surgically by routing the right ventricle to aorta (VSD is approached from the aortic root) along with arterial switch operation or routing left ventricle to aorta (VSD is approached from the right atrium) along with pulmonary root translocation.9) While the former option is reported rarely, the more commonly practiced root translocation is more invasive. Subaortic VSD with normal great artery relation favoured a closure by routing the right ventricle to the aortic root in the first patient, who received concurrent arterial switch for correcting the circulation.9)
Surgery in patients with unobstructed pulmonary valve also depends on the malposition of great arteries and the age of the patient. If aorta is anterior and malposed, surgery involves an intraventricular tunneling of left ventricle to aorta followed by pulmonary root translocation.10) If young infants are too small for a root translocation, pulmonary artery banding is offered.11) In the rare form of subpulmonary VSD without pulmonary stenosis, a simple routing of the right ventricle to the pulmonary artery corrects the entire anatomy.6)
Pulmonary stenosis is very commonly seen in 90% of the cases.1, 3) The second patient had severe pulmonary stenosis. Presence of two good-sized ventricles with normal atrioventricular valves is common in DOLV and favours biventricular repair as seen in our second patient. Patients with hypoplastic right ventricle and tricuspid valve are managed by univentricular staged surgeries.3, 4)
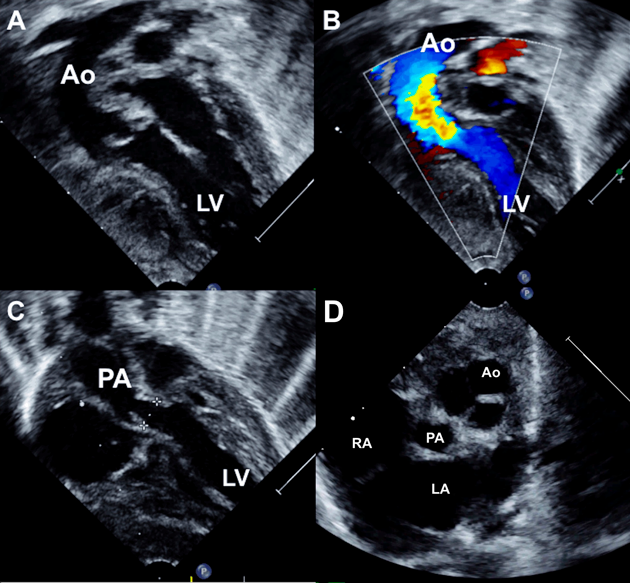
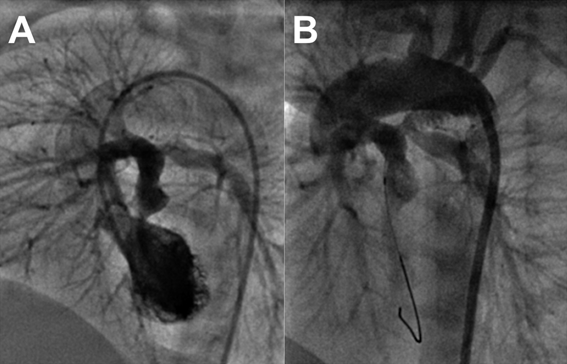
Surgery in DOLV in the presence of pulmonary stenosis depends on the age of the patient. Commonest repair option in patients with subaortic VSD beyond infancy is Rastelli surgery to route left ventricle to aorta and connect pulmonary artery to right ventricle through an extracardiac valved conduit.7) Pulmonary artery translocation after suture closing the stenosed pulmonary valve is another option if conduits are unavailable. Small infants may be palliated temporarily till definitive surgery. Provision of additional pulmonary blood flow may be achieved with an aortopulmonary shunt or percutaneous stenting of the ductus arteriosus. Improvement of intercirculatory mixing to prevent unfavourable streaming may be achieved by surgical atrial septectomy or percutaneous balloon atrial septostomy. One additional patient in our center with DOLV, subaortic VSD, d-malposed aorta was initially palliated in neonatal period with ductal stenting and balloon atrial septostomy (Fig. 6) followed later at one year with Rastelli surgery.
Double outlet left ventricle is most commonly associated with subaortic ventricular septal defects, but may present with large anatomical variations. Patients with pulmonary stenosis have severe cyanosis due to additional unfavourable streaming and clinical presentation is earlier than Fallot’s tetralogy. Patients with unobstructed pulmonary flows show transposition physiology. Treatment algorithms depend on the age of the patient, location of the ventricular septal defect, presence of pulmonary stenosis, great artery relationship, availability of conduits and adequacy of the right ventricle.
引用文献References
1) Tchervenkov C, Walters HL 3rd, Chu VF: Congenital heart surgery nomenclature and database project: Double outlet left ventricle. Ann Thorac Surg 2000; 69 Suppl: S264–S269
2) Anderson R, Galbraith R, Gibson R, et al: Double outlet left ventricle. Br Heart J 1974; 36: 554–558
3) Shanmugam G, Pollock J, Macarthur K: Double outlet left ventricle, transposition of great vessels and pulmonary stenosis. Asian Cardiovasc Thorac Ann 2004; 12: 159–161
4) Imai-Compton C, Elmi M, Manlhiot C, et al: Characteristics and outcomes of double outlet left ventricle. Congenit Heart Dis 2010; 5: 532–536
5) Menon SC, Hagler DJ: Double-outlet left ventricle: Diagnosis and management. Curr Treat Options Cardiovasc Med 2008; 10: 448–452
6) Sakakibara S, Takao A, Arai T, et al: Both great vessels arising from the left ventricle. Bull Heart Institute Jpn 1967: 66
7) Hagler DJ: Double Outlet left ventricle. Allen HD, Driscoll DJ, Shaddy RE, et al (eds): Moss & Adams Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young. 7th edition. Baltimore. Lippincott Williams & Wilkins, 2008, pp1120-1127
8) Paul MH, Muster AJ, Sinha SN, et al: Double-outlet left ventricle with an intact ventricular septum: Clinical and autopsy diagnosis and developmental implications. Circulation 1970; 41: I29
9) Varghese R, Arora N, Sherrif EA, et al: Arterial switch operation for double-outlet left ventricle. Ann Thorac Surg 2014; 98: e97–e99
10) Ootaki Y, Yamaguchi M, Oshima Y, et al: Pulmonary root translocation for biventricular repair of double-outlet left ventricle. Ann Thorac Surg 2001; 71: 1347–1349
11) Gouton M, Bozio A, Rey C, et al: Double outlet left ventricle: A rare and unusual cardiopathy. Apropos of 7 new cases. Arch Mal Coeur Vaiss 1996; 89: 553–559 (in French)