Pulmonary artery (PA) sling is a rare vascular anomaly wherein the left PA arises from the right PA, and the left PA runs toward the left between the lower trachea and esophagus. PA sling is often associated with congenital tracheal stenosis. Complete tracheal rings are often found in PA sling patients, and also referred to as “ring-sling” complex. External compression and intrinsic stenosis of the trachea lead to respiratory symptoms. Depending on its severity it may present with simple stridor or with near-death episodes requiring resuscitation. The natural history of PA sling is a poor outcome because of airway obstruction.1) Current surgical management of symptomatic PA sling involves reimplantation of the anomalous left PA and often concomitant repair of tracheal stenosis.2) However, tracheal surgery requires a large amount of surgical expertise with a certain degree of morbidity and mortality.3) Moreover, long-segment or distal tracheal stenosis involving the carina is life-threatening and difficult to treat. The treatment for long-segment tracheal stenosis remains challenging and is still controversial. In this study, we aimed to review our all consecutive surgical experience of PA sling, and to evaluate the degree of tracheal growth after the PA sling repair without tracheoplasty.
Patients
The medical institutional review board at Japan Community Health Care Organization (JCHO) Kyushu Hospital approved this study. From August 2006 to August 2015, a total of consecutive six patients including two boys and four girls underwent PA sling repair at JCHO Kyushu Hospital. The median age was 6.7 months (range, 2.7–21.7 months), and the median weight was 7.1 kg (range, 3.3–8.2 kg). The patients’ characteristics are shown in Table 1. All patients had symptoms of tracheal stenosis ranging from mild stridor to severe respiratory distress. All patients had the following coexisting cardiac anomalies: patent ductus arteriosus (PDA) in four, patent foramen ovale in three, ventricular septal defect (VSD) in three, a left superior vena cava in two, atrial septal defect (ASD) in one, a partial anomalous pulmonary venous connection (PAPVC) in one, and right PA stenosis in one patient, respectively. Additionally, two patients had tracheal bronchus, two had tracheomalacia, two had a hypoplastic right lung, and two had a tracheal ring. Tracheal ring was evaluated by bronchoscopy. Two of six patients required preoperative mechanical respiratory support. No patient had previous cardiac and tracheal intervention before PA sling surgery. In all six patients, preoperative contrasted computed tomography (CT) was performed to evaluate the degree of tracheal stenosis and state of PA sling. Preoperative cardiac catheterization was performed in five patients.
Table 1 Patients’ preoperative characteristics| Characteristics | Patients number |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Age (month) | 4.6 | 8.7 | 9.5 | 2.9 | 2.7 | 21.7 |
| Weight (kg) | 6.8 | 7.3 | 7.9 | 3.3 | 3.8 | 8.2 |
| Sex | M | F | M | F | F | F |
| Symptoms | Stridor | Stridor | Stridor | Tachypnea | Stridor | Stridor |
| Associated anomalies | VSD, PDA, LSVC | PFO, LSVC | PFO | VSD, ASD, PDA, PAPVC | VSD, PFO PDA, RPAS | PDA |
| Preoperative ventilation | + | − | − | − | + | − |
| Narrowest diameter (mm) | 1.5 | 2.3 | 2.8 | 2.1 | 2.1 | 2.1 |
| Stenotic segment ratio (%) | 57 | 51 | 31 | 60 | 59 | 63 |
| Type of tracheal stenosis | Long | Long | Long | Long | Long | Short |
| Longitudinal stenotic tracheal segment ratio (%) | 80 | 61 | 83 | 75 | 84 | 38 |
| Tracheal ring | − | − | − | + | + | − |
| Tracheal bronchus | − | − | + | − | − | + |
| Tracheomalacia | + | − | − | − | + | − |
| Right lung hypoplasia | − | − | + | − | + | − |
| F=female, M=male. ASD: atrial septal defect, LSVC: left superior vena cava, PAPVC: partial anomalous pulmonary venous connection, PDA: patent ductus arteriosus, PFO: patent foramen ovale, RPAS: right pulmonary artery stenosis, VSD: ventricular septal defect. |
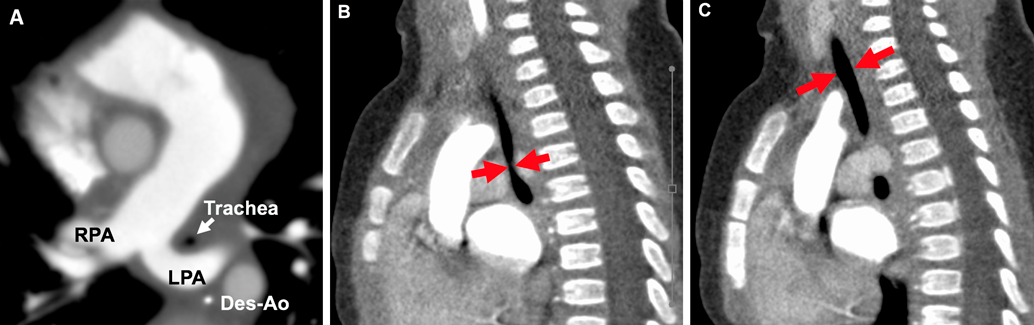
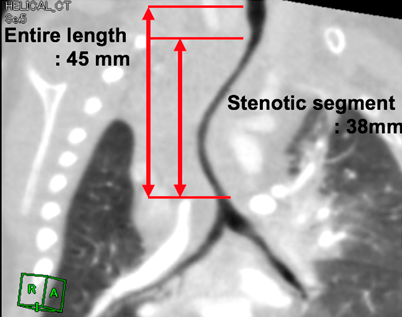
We evaluated the details of tracheal stenosis using CT (Fig. 1A) and calculated the stenotic segment ratio, which was estimated by the following formula (Fig. 1B, 1C)4):We also calculated the longitudinal stenotic tracheal segment ratio as shown in Fig. 2. Long-segment tracheal stenosis was defined as the ratio of the stenotic tracheal segment to that of the entire tracheal length of >50%.5)
In our institution, accompanying tracheoplasty for the PA sling repair was not a routine surgical procedure at that time.
Operative Technique
All of the patients underwent one-stage total correction for PA sling repair and concomitant intracardiac anomalies without tracheoplasty through median sternotomy under cardiopulmonary bypass. Aortic cross-clamping was undertaken in all patients. The anomalous left PA was cut away from the right PA and moved anterior to the trachea. Thereafter, we reimplanted the anomalous left PA onto the main PA in an end-to-side fashion.
Data Analysis
Statistical analyses were performed using R 3.5.2 (CRAN: the Comprehensive R Archive Network at http://cran.r-project.org/). A p-value <0.05 was considered statistically significant.
Early Postoperative Course
Operative and postoperative characteristics are shown in Table 2. At the time of PA sling repair, six patients had a concomitant cardiac surgical procedure. There was no operative death. Before extubating, we checked the degree of tracheal stenosis in the operating room or intensive care unit with bronchoscopy. The postoperative intubation time ranged from 1 to 19 days (median: 7 days). A total of two patients required reintubation during their hospital stay after the first extubation. Thereafter, all six patients were discharged without respiratory difficulty. The median postoperative hospital stay was 29.5 days.
Table 2 Patients’ operative and postoperative characteristics| Characteristics | Patients number |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Operation | Reimplantation | Reimplantation | Reimplantation | Reimplantation | Reimplantation | Reimplantation |
| Duration of postoperative intubation (days) | 19 | 1 | 13 | 7 | 7 | 4 |
| Reintubation | − | − | + | + | − | − |
| Reintervention | None | None | None | None | BAP | None |
| Outcome | Asymptomatic | Asymptomatic | Asymptomatic | Late death | Asymptomatic | Mild exercise intolerance |
| BAP: balloon angioplasty. |
Follow-Up and Reintervention
The follow-up periods ranged from 5.5 to 14.5 years (median: 10.5 years). There was one late death in patient No. 4 who was diagnosed with VSD, ASD, PDA, PAPVC, and PA sling with long-segment tracheal stenosis. The cause of late death was pneumonia, leading to eventual respiratory failure 6 months after the initial PA sling repair.
Left PA patency was assessed in all patients by contrast CT and cardiac catheterization. The left PA was patent in all patients. However, only patient No. 5 had left PA stenosis at the anastomosis site and required catheter balloon angioplasty (BAP) at 7 months after the PA sling operation. The last follow-up cardiac catheterization at 3.5 years after the BAP showed no major residual left PA stenosis.
Tracheal Growth
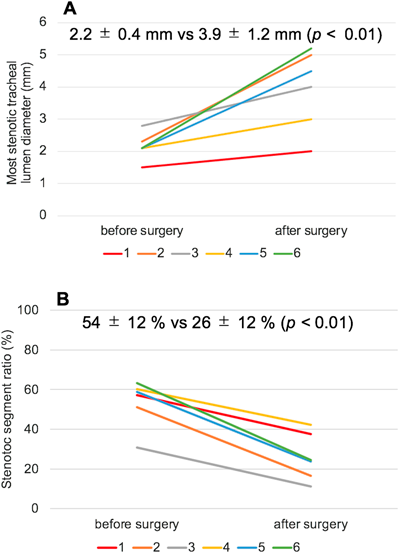
Postoperative tracheal lesions were assessed using contrast CT in all patients. The preoperative median stenotic segment ratio and longitudinal stenotic tracheal segment ratio were 54% and 78%, respectively. In five of six patients, the longitudinal stenotic tracheal segment ratio was >50%. All patients showed significant growth in the tracheal lumen diameter (Fig. 3A). The mean lumen diameter of the most stenotic part of the trachea increased from 2.2 mm before to 3.9 mm after PA sling repair (p<0.01, paired t test). Additionally, the stenotic segment ratio improved from 54% before to 26% after PA sling repair (p<0.01, paired t test) (Fig. 3B).
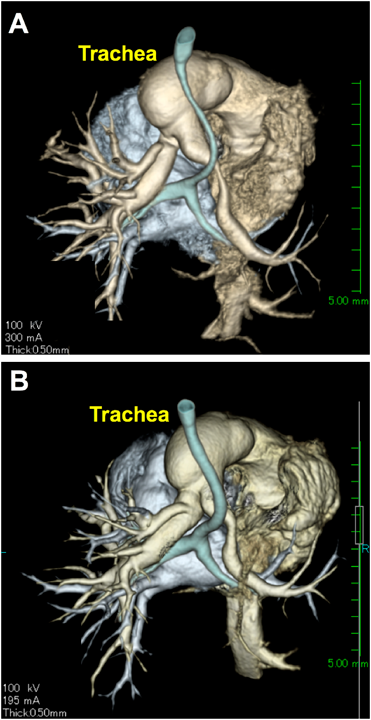
Although, in some patients, the trachea showed a certain degree of residual tracheal stenosis compared to the normal diameter on the last follow-up CT, the degree of stenosis was improved, and the other patients showed excellent tracheal growth (Fig. 4A, 4B).
Fig. 4 showed the postoperative three-dimensional contrast CT in patient No. 5 at 2 weeks (A) and 1 year (B) after the surgery. The patient No. 5 had a very long longitudinal stenotic tracheal segment ratio of 84%, however, the longitudinal stenosis was clearly improved at 1 year after the surgery. All 5 surviving patients have not experienced any major respiratory problems, fortunately. Also, no patients have experienced any tracheal intervention after the PA sling repair.
In our study, operative and late mortality rates after PA sling repair without tracheoplasty were 0% and 16.6% (1/6), respectively. Previously reported early and late mortality rates after PA sling repair ranged from 0% to 17% and 0% to 21%, respectively.6) Moreover, only one patient in our series developed left PA stenosis, and BAP was required 7 months after the initial operation. Previous studies on PA sling also reported a good patency rate.7, 8)
Our initial concern was whether tracheal stenosis would improve by PA sling repair only. Fortunately, a retrospective review of our series demonstrated that all patients showed significant tracheal growth soon after the repair. However, it is difficult to define absolute tracheal surgical indication based on either tracheal size or severity of symptoms.
Anton-Pacheco et al.9) observed patients with mild stenosis without tracheal intervention. Additionally, Van Son et al.10) stated that conservative treatment was preferable for mild or moderate tracheal stenosis. Although the definition of “severe symptoms” is ambiguous, most of the published data on congenital tracheal stenosis with PA sling describe cases with “complete tracheal rings and severe symptoms”, which are treated surgically.11) Backer et al.8) suggested if PA sling patients exhibited complete tracheal rings, tracheoplasty was required. However, Elliott et al.12) stated that congenital tracheal stenosis is not synonymous with complete tracheal rings. They observed that a number of patients with PA sling and complete tracheal ring did survive without tracheoplasty.
Few reports have described the growth of the trachea after the PA sling repair. Healey et al. had used 2 mm as a critical lower limit of internal diameter, although no objective evidence appears to be present to justify this number.13) Huang et al.14) found that in children with PA sling repair without tracheoplasty, the diameter of the trachea on follow-up CT images showed interval growth. They found that the clinical outcome was poor without tracheoplasty when the diameter of the trachea was less than 3 mm. Therefore, they proposed that if PA sling patients had the diameter of the trachea of <3 mm, tracheoplasty and PA sling repair were needed.
Since the first reports of tracheal stenosis repair in 1982,15) various tracheal repair techniques have been proposed. Over the last decade, slide tracheoplasty, as described by Tsang et al.,16) has emerged as the technique of choice for repair of tracheal stenosis. In high-volume centers, survival outcomes after slide tracheoplasty are excellent. Butler et al.17) and Manning et al.18) reported an early mortality rate of 6% and 2.5%, respectively. Slide tracheoplasty with PA sling surgery also has good results. Oshima et al.7) reported an early mortality rate of 6.5% after PA sling repair in 31 patients, of whom 28 underwent tracheoplasty. Similarly, Backer et al.8) reported no early mortality after PA sling repair in 34 patients of whom 26 underwent tracheoplasty. Speggiorin et al.19) demonstrated tracheal growth after slide tracheoplasty using bronchography investigations. However, the tracheal surgery has several possible postoperative complications, such as progressive growth of granulation tissue at the anastomosis site that might require future reoperation or reintervention, air leakage that can induce lethal problems in the postoperative recovery period, and injury of nerves or vessels around the trachea.20) Despite several studies reporting tolerance and acceptable overall mortality after accompanying tracheal surgery, patients required a certain number of airway reoperation or reintervention. Yong et al.6) reported that significant granulation tissue developed in seven (33.3%) patients after tracheal surgery and it required multiple interventions. Even in high-volume centers, the rate of any airway reoperation or reintervention after slide tracheoplasty ranges between 29% and 48%.17, 18)
The ideal treatment for long-segment tracheal stenosis remains controversial, although most surgeons perform tracheoplasty for diffuse or severe tracheal stenosis. The indications for tracheal surgery in patients with PA sling vary from institution to institution. In our institution, accompanying tracheoplasty was not a routine surgical procedure at that time. However, when we retrospectively reviewed the patient No. 4 who was a late death case, the patient No. 4 did not acquire enough improvement compared to the other patients as shown in Fig. 3B. The patient No. 1 also showed insufficient tracheal growth and he required 19 days postoperative mechanical ventilation period. Reflecting our results, awareness of the PA sling, and tracheal problems, our current policy is to consult the PA sling patients to the other facility accustomed to tracheal surgery prior to the PA sling repair as much as possible. The patient No. 1 and No. 5 had the VSD, and both of them had secondary tracheomalacia due to dilated main pulmonary artery compression, which caused by severe pulmonary hypertension. We think that an enlarged main pulmonary artery gradually became smaller after closure of the VSD, which might contribute to the tracheal growth.
As mentioned above, a retrospective review of our series showed significant tracheal growth after the PA sling repair without tracheoplasty despite in all six patients the narrowest tracheal diameter was about 2–3 mm and five patients had long-segment tracheal stenosis. Although the trachea remained stenotic compared to the normal diameter, none of the survivors had major respiratory problems, and they did not require an additional tracheal procedure for their airway. However, as respiratory infection is very risky like patient No. 4, aggressive management of upper respiratory infection and close follow-up evaluation should be mandatory for these patients.
This study was limited by its retrospective nature, single-center design, the small sample size, and the short follow-up period. Because of the small number of patients in our study, we could not verify some statistical outcomes from our data. Additionally, we have had a smaller proportion of patients with a complete tracheal ring compared to the previous reports. Multicenter studies with a large sample size are warranted.
The surgical outcomes for PA sling without tracheoplasty were acceptable in our patients, and the survivors remained asymptomatic with sufficient tracheal growth. More studies will be needed to understand the long-term outcomes for repaired and unrepaired tracheal stenosis in patients with PA sling.
謝辞Acknowledgments
We thank Ellen Knapp, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
All authors have no conflict of interest.
引用文献References
1) Backer CL, IIbawi MN, Idriss FS, et al: Vascular anomalies causing tracheoesophageal compression: Review of experience in children. J Thorac Cardiovasc Surg 1989; 97: 725–731
2) Yong MS, D’Udekem Y, Brizard CP, et al: Surgical management of pulmonary artery sling in children. J Thorac Cardiovasc Surg 2013; 145: 1033–1039
3) Wei C, David EM, Victor F, et al: The role of conservative management in congenital tracheal stenosis: An evidence-based long-term follow-up study. J Pediatr Surg 2006; 41: 1203–1207
4) Tanimoto T, Bitoh Y, Okata Y, et al: Consevative management in congenital tracheal stenosis: Clinical course after long-term follow-up. J Jpn Soc Pediatr Surg 2019; 55: 248–252 (in Japanese)
5) Terada M, Hotoda K, Kotani S, et al: Slide tracheoplasty for congenital tracheal stenosis: Experience with 16 cases. J Pediatr Cardiol Card Surg 2009; 25: 608–615 (in Japanese)
6) Yong MS, Zhu MZL, Bell D, et al: Long-term outcomes of surgery for pulmonary artery sling in children. Eur J Cardiothorac Surg 2019; 56: 369–376
7) Oshima Y, Yamaguchi M, Yoshimura N, et al: Management of pulmonary artery sling associated with tracheal stenosis. Ann Thorac Surg 2008; 86: 1334–1338
8) Backer CL, Russell HM, Kaushal S, et al: Pulmonary artery sling: Current results with cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012; 143: 144–151
9) Anton-Pacheco JL, Cano I, Comas J, et al: Management of congenital tracheal stenosis in infancy. Eur J Cardiothorac Surg 2006; 29: 991–996
10) Van Son JA, Hambsch J, Haas GS, et al: Pulmonary artery sling: Reimplantation versus antetracheal translocation. Ann Thorac Surg 1999; 68: 989–994
11) Backer CL, Mavroudis C, Gerber ME, et al: Tracheal surgery in children: An 18-year review of four techniques. Eur J Cardiothorac Surg 2001; 19: 777–784
12) Elliott M, Roebuck D, Noctor C, et al: The management of congenital tracheal stenosis. Int J Pediatr Otorhinolaryngol 2003; 67Suppl 1: S183–S192
13) Manson D, Filler R, Gordon R: Tracheal growth in congenital tracheal stenosis. Pediatr Radiol 1996; 26: 427–430
14) Huang SC, Wu ET, Wang CC, et al: Surgical management of pulmonary artery sling: Trachea diameter and outcomes with or without tracheoplasty. Pediatr Pulmonol 2012; 47: 903–908
15) Ein SH, Friedberg J, Williams WG, et al: Tracheoplasty: A new operation for complete congenital tracheal stenosis. J Pediatr Surg 1982; 17: 872–878
16) Tsang V, Murday A, Gillbe C, et al: Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg 1989; 48: 632–635
17) Butler CR, Speggiorin S, Rijnberg FM, et al: Outcomes of slide tracheoplasty in 101 children: A 17-year single center experience. J Thorac Cardiovasc Surg 2014; 147: 1783–1789
18) Manning PB, Rutter MJ, Lisec A, et al: One slide fits all: The versatility of slide tracheoplasty with cardiopulmonary bypass support for airway reconstruction in children. J Thorac Cardiovasc Surg 2011; 141: 155–161
19) Speggiorin S, Gillbert TW, Broadhead M, et al: Do tracheas grow after slide tracheoplasty? Ann Thorac Surg 2012; 93: 1083–1086
20) Kwak JG, Kim WH, Min J, et al: Is tracheoplasty necessary for all patients with pulmonary artery sling and tracheal stenosis? Pediatr Cardiol 2013; 34: 498–503