Williams syndrome (WS) is a rare genetic disorder characterized by cardiovascular diseases, an elfin-like face, dental abnormalities, urinary tract anomalies, and mental retardation. The detection of microdeletions at chromosome 7q11.23, encompassing the elastin (ELN) gene, establishes the diagnosis. The prevalence of cardiac anomalies in WS is reported to be 80%, including peripheral pulmonary artery stenosis, supravalvular aortic stenosis (SAS), and coronary artery stenosis.1–3) Mortality depends on the severity of these cardiovascular anomalies. Cases of infective endocarditis (IE) with SAS have been reported in the literature.4–6) However, to the best of our knowledge, there have been only two reports of IE associated with mitral regurgitation in patients with WS.6, 7)
Herein, we report two WS cases with IE associated with mitral regurgitation.
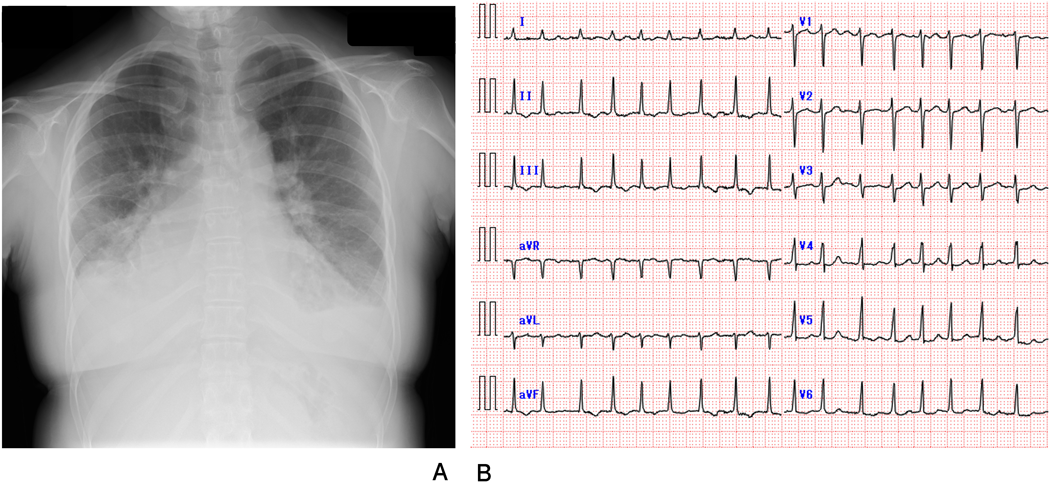
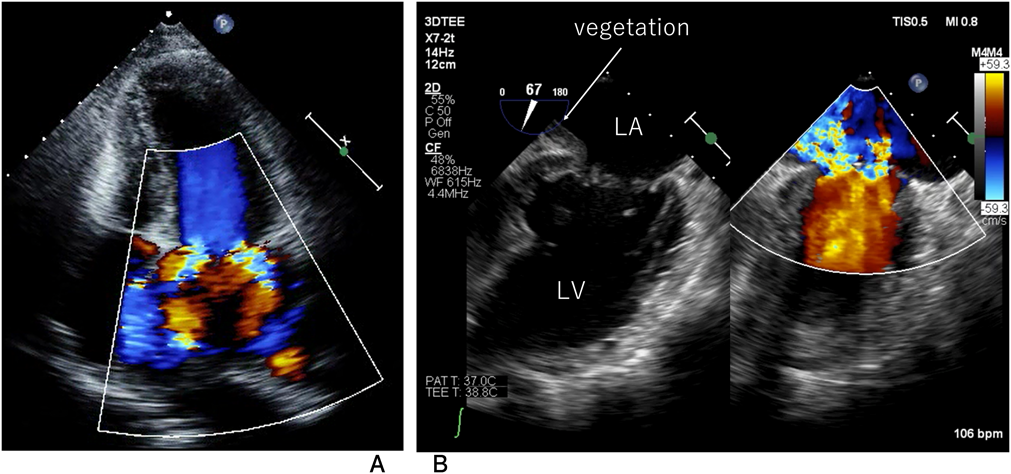
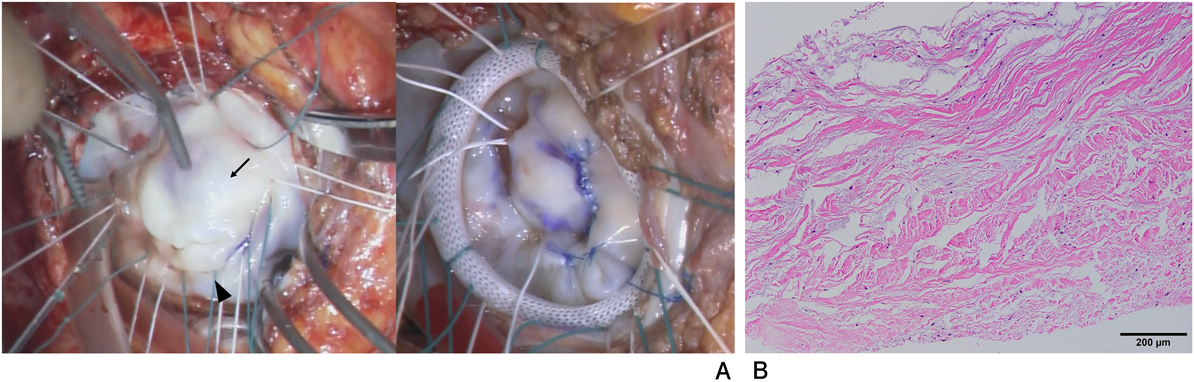
A 30-year-old woman was diagnosed with WS due to a characteristic face and SAS after birth. The Doty procedure was performed at the age of 5 years. Although SAS was relieved, mitral valve prolapse and mitral regurgitation were detected at the age of 10 and progressed to a grade of III at the age of 20. She presented with mental retardation associated with behavioral problems. Removal of teeth plaque was required every 3 months because of malocclusion and dental caries. She was admitted to our hospital because of a remittent fever one month before admission and dyspnea one week before admission after a dental procedure without prior administration of prophylactic antibiotics. On admission, she had a high-grade fever, dyspnea, her eyelids and bilateral lower legs were markedly edematous, and she displayed weight gain of 10 kg in 2 weeks. The resting heart rate was irregular, and auscultation revealed a grade 2/6 regurgitant systolic murmur at the apex. Complete blood counts showed a white blood cell level (WBC) of 1.10×1010/L, a hemoglobin level of 12.5 g/dL, and a platelet level of 143×109/L. The levels of C-reactive protein (CRP) and B-type natriuretic peptide were 0.52 mg/dL (normal range<0.14 mg/dL) and 352.7 pg/mL (normal range<18.4 pg/mL), respectively. Chest radiography showed cardiomegaly, bilateral pulmonary congestion, and bilateral pleural effusion (Fig. 1A). Electrocardiography revealed atrial fibrillation that had never been diagnosed (Fig. 1B) and transthoracic echocardiography revealed left atrium dilatation, mitral valvular anterior leaflet prolapse, and severe mitral regurgitation (Fig. 2A). In transesophageal echocardiography, isoechoic vegetation was identified on the left side of the atrial septum, the impact site of the mitral regurgitant stream (Fig. 2B). Two sets of blood culture were positive for Streptococcus gordonii, which is sensitive to penicillin G and ampicillin. Electrical cardioversion (100 J) restored sinus rhythm, and the patient was subsequently treated with intravenous ampicillin and gentamicin. She became afebrile the next day, and blood cultures were sterile on day 12 of hospitalization. WBC and CRP levels normalized by day 27, and the vegetation disappeared completely on day 43. Antibiotic administration was discontinued on day 44. The degree of mitral regurgitation remained severe despite the improvement in symptoms and laboratory data. Consequently, we performed mitral annuloplasty, pulmonary vein isolation, and left appendage closure 3 months after admission. The anterior mitral leaflet was large and thick, and the posterior leaflet was smaller in dimension (Fig. 3A). An incision was made on a part of the anterior mitral leaflet, and plication of the bilateral commissure was performed. The resected specimen of the mitral valve showed minimal chronic inflammation with myxoid degeneration (Fig. 3B). She was discharged on day 14 after surgery without any complications.
A 34-year-old woman was diagnosed with WS and supravalvular aortic/pulmonary artery stenosis after birth, which had not required surgery. She was diagnosed with enamel hypoplasia in childhood without regular dental care. She did not interfere with her daily life and was a part-time worker doing cleaning work. She was pointed out to have cardiac hypertrophy in her medical checkup at work at the age of 30 years and mild mitral regurgitation was detected. She was referred to our hospital because of remittent fever and lower back pain unresponsive to oral antibiotics. White blood cell count, hemoglobin level, and platelet count were 1.65×1010/L, 12.4 g/dL, and 227×109/L, respectively. The level of CRP was 25.7 mg/dL. Urinalysis showed pyuria. Two sets of blood culture grew methicillin-susceptible Staphylococcus aureus. Transthoracic echocardiography demonstrated mitral anterior leaflet prolapse and moderate mitral regurgitation with isoechoic vegetation on the mitral posterior leaflet. Abdominal ultrasonography and contrast-enhanced computed tomography did not show obvious urethral malformations. She was treated with intravenous cefazolin and gentamicin and became afebrile on day 4 of hospitalization. The vegetation gradually disappeared. On day 28, she was discharged with moderate mitral regurgitation. However, NYHA class was 2 at the age of 35, which became more severe at the age of 38. We performed mitral valve repair at the age of 40 years. After three years, she has not presented with symptoms of heart failure.
We present two WS cases with IE due to mitral regurgitation. The present report suggests patients with WS are at higher risk for IE than those without WS because of their cardiac and extracardiac complications including dental abnormalities. Supra aortic stenosis is the most common cardiac anomaly in WS, with a prevalence of approximately 45–75%.1, 2) Mitral valve prolapse and regurgitation have been reported in 15% of patients with the syndrome.8) While these lesions do not require surgery or intervention in most cases, some cases show the progression of mitral regurgitation during adulthood.3, 8, 9) If patients with WS have mitral valvular prolapse, examination by echocardiography should be repeated at least once a year.
Katan et al. predicted the occurrence of IE in 1 of approximately 340 patients with MVP and >moderate MR per year or a 0.3% patient-year risk.10) Although the frequency of IE in patients with WS has been unclear, some previous reports have described the association of IE with SAS in patients with WS.4–6) On the other hand, there have been only two case reports of IE associated with mitral valve prolapse and mitral regurgitation in WS.6, 7) Indeed, a cohort study reported that 3 (5.7%) of 52 patients with WS developed IE.11) These reports could reveal WS are at a potential high risk of IE.
Elastin arteriopathy is considered to be the primary cause of SAS, peripheral pulmonary artery stenosis, and coronary artery stenosis in patients with WS.2) Vascular media with a proliferation of hypertrophied muscle cells increase collagen content, and reduced elastic tissue in the form of broken and disorganized elastin fibers might be responsible.12, 13) Although histological findings of the mitral valve are not fully understood in WS, our case demonstrated myxoid degeneration and collagenous changes in the mitral valve leaflet, consistent with aortic lesions.15) There might be a histological association between the mitral valve and aortic lesion in patients with WS.
A high velocity jet is one of the predisposing factors for IE eliciting injury to the endothelium, resulting in the formation of nonbacterial thrombotic endocarditis on the surface of the damaged endothelium. In addition, bacterial colonization might evoke negative cycles of endothelial injury and thrombus deposition, finally forming vegetation. The vegetation sites, in the present cases, were detected at the left atrial wall and mitral posterior leaflet, which were the end and origin of the turbulent flow. Consequently, the mitral regurgitation jet should be associated with the onset of IE.
Streptococcus gordonii is a commensal organism of the oral and gastrointestinal tracts. IE might have been induced by dental procedures in Case 1. Almost all patients with WS have dental anomalies, including malocclusion, hypodontia, malformed teeth, and enamel hypoplasia.1) These problems are related to the high prevalence of dental caries requiring regular supportive dental care. However, these patients are exposed to frequent bacteremia by these repeated dental procedures, triggering IE. In the JCS 2017 guidelines,15) prophylactic antibiotic recommendations for patients with mitral regurgitation among adult patients with congenital heart disease are not an absolute indication. However, the present report suggests that prophylactic antibiotics should be recommended for WS having MR and dental abnormalities at dental procedure including removing plaque.
In Case 2, although urinalysis showed pyuria, the urine culture was negative. There is no evidence that served as an entry gate for methicillin-susceptible Staphylococcus aureus. On the other hand, WS is likely to involve urinary tract anomalies and urinary tract infections (UTIs)1, 3) resulting in bacteremia. Thus, UTI may be a risk factor for IE in WS.