The subjects were those who were consulted to our team by doctors from other hospitals about lymphatic disease May 2016 to April 2020. Only central lymphatic disease consultations were included, with symptoms of chylothorax, chylous ascites, pericardiac effusion, and protein-losing enteropathy. Those limited to peripheral lymphatic disorders (lymphedema and/or lymphangioma) were excluded. A series of exchanges were counted as single consults; however, different cases or other questions from previous consultants were counted as independent consults. Detailed non-trackable consults were excluded, such as phone calls that were not recorded.
We classified consultant regions (Hokkaido, Tohoku, Kanto, Chubu, Kinki, Chugoku, Shikoku, Kyusyu-Okinawa), characteristics of consulting hospitals (University hospital, Specialty hospital, General hospital), consultant physician’s specialty, cases (age, anomalies, genetic diseases, symptoms, previous surgeries), contents of the consult (operation request, treatment plan, testing procedure, medical treatment, nutrition), and our responses. The contents of the consults were counted individually when they included more than two questions in the first e-mail, but the additional questions in the following e-mails in the series consult were not included.
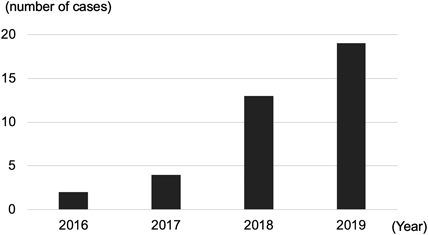
A total of 47 consults were collected during a period of 4 years (48 months). Nine consults were excluded from this study because they were not linked with central lymphatic disease; therefore, 38 cases were included. The number of consults increased every year, with 19 consults (out of 38 consults, 50%) in last year, 2019 (Fig. 1).
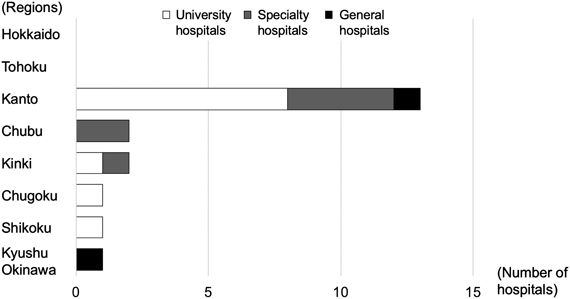
Consultants were affiliated to 20 hospitals. Regional distribution showed that more than half of the hospitals were in Kanto region (13 of 20 hospitals, 65%), but there was at least one hospital from all regions west to Chubu. Hospital type classification indicated that university hospitals were the most common (11 out of 20, 55%), followed by specialty hospitals (specialized for pediatric hospitals or cardiovascular hospitals) (7, 35%), and general hospitals (2, 10%) (Fig. 2).
Consultation numbers from each hospital varied; there were single consultations from 11 hospitals (out of 20, 55%), while nearly half 9 hospitals (out of 20, 45%), consulted twice or more. Of the hospitals that consulted more than twice, the majority were in the Kanto region (7 hospitals out of 9, 78%), and one hospital each was in Chubu and Kinki.
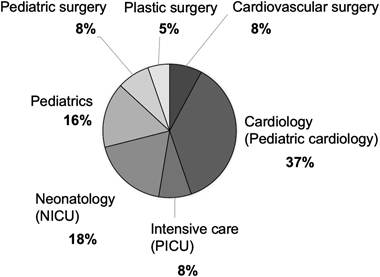
A total of 17 consulters (out of 38, 45%) specialized in the cardiovascular field (pediatric cardiology, pediatric cardiovascular surgery), with pediatric cardiology being the most common (14 consulters, 37%), followed by specialists in the intensive care field (intensive care unit, neonatology) (10, 26%) and pediatric field (pediatrics, pediatric surgery) (9, 24%), with consulters from plastic surgery being the least (2, 5%) (Fig. 3).
Consultations were done for a total of 37 cases, with one case in each of the 38 consults except for one. The majority of the cases were infants; almost half were less than 3 months old (18 cases out of 38, 49%) (overall range was from 2 days to 18 years old, mean age was 2 years 6 months).
Chylothorax was the major symptom in the consults, seen as the presenting symptom in 33 cases out of 37 (89%), followed by chylous ascites (6 cases, 16%), and general edema (4 cases, 11%). Several cases showed chylous pericardial effusion and protein-losing enteropathy. Background conditions of congenital heart diseases were seen, with hypoplastic left heart syndrome being the most common (11 cases out of 37, 30%), followed by total anomalous pulmonary venous return and coarctation of aorta (4 cases, 11%). Many cases underwent cardiovascular surgery prior to the consult (28 cases out of 37, 76%); however, some cases did not undergo previous surgeries or did not have congenital anomaly (Table 1).
Table 1 List of consult cases| No | Age | Malformations | Genetic disorder | Symptoms | Surgeries | Consult purpose |
|---|
| 1 | 0 M | Cardiac (detail unknown) | 21trisomy | PE, Ascitis, Edema | PDA ligation | Plan, Test |
| 2 | 0 M | | | PE, Edema | | Operation, Plan |
| 3 | 0 M | CoA | Noonan | PE | CoA repair | Plan, Test |
| 4 | 0 M | HLHS | | PE | PA banding | Operation |
| 5 | 1 M | HLHS | | PE, Ascitis | Cardiac (detail unknown) | Operation, Plan |
| 6 | 1 M | | | PE, Edema | | Operation |
| 7 | 1 M | Cardiac (detail unknown) | 21trisomy | PE, Edema | PDA ligation | Operation, Plan |
| 8 | 1 M | Cardiac (detail unknown) | | PE | PA banding | Plan, Test |
| 9 | 1 M | | 21trisomy | PE | | Plan, Test |
| 10 | 1 M | Cardiac (detail unknown) | | PE | Cardiac (detail unknown) | Plan, Test |
| 11 | 1 M | | | PE | | Plan |
| 12 | 1 M | | | PE | | Neutrition |
| 13 | 1 M | | | Ascitis | | Operation, Plan |
| 14 | 2 M | Cardiac (detail unknown) | | PE, Ascitis | Cardiac (detail unknown) | Plan |
| 15 | 2 M | esophageal atresia | | PE | Esophageal atresia repair | Test |
| 16 | 2 M | HLHS | | PE | Norwood | Operation |
| 17 | 2 M | TAPVR, esophageal atresia | | PE | TAPVR repair | Operation |
| 18 | 2 M | CoA | | PE | CoA repair | Operation |
| 19 | 2 M | Ebstein, Cardiac (detail unknown) | | PE | Cardiac (detail unknown) | Operation |
| 20 | 2 M | TAPVR | | PE | TAPVR repair | Operation |
| 21 | 3 M | HLHS | | PE | Norwood | Operation, Plan |
| 22 | 3 M | Cardiac (detail unknown) | | PE | Cardiac (detail unknown) | Operation |
| 23 | 3 M | Cardiac (detail unknown) | | PE | PDA ligation | Operation, Plan |
| 24 | 3 M | | | PE | | Operation |
| 25 | 3 M | esophageal atresia | 18trisomy | Ascitis, Pericardial | PDA ligation, PA banding | Plan, Test |
| 26 | 5 M | HLHS | | PE | Glenn | Plan |
| 27 | 6 M | HLHS | | PE | Norwood, PA banding | Plan, Test |
| 28 | 9 M | Cardiac (detail unknown) | | PE, Pericardial | Rastelli | Operation |
| 29 | 1 Y | | | Ascitis | | Test |
| 30 | 1 Y | TAPVR | | PE | TAPVR repair | Operation |
| 31 | 2 Y | polyductyle | | PE | CoA repair | Operation, Plan |
| 32 | 2 Y | HLHS, TAPVR | | PE | Fontan | Test |
| 33 | 5 Y | CoA | | PE | CoA repair | Plan |
| 34 | 7 Y | HLHS | | PE | Fontan | Plan, Test |
| 35 | 9 Y | HLHS | | PE | Fontan | Plan, Test |
| 36 | 13 Y | HLHS | | PE | Fontan | Operation, Plan |
| 37 | 18 Y | HLHS | | PLE | Fontan | Plan |
| The consult purposes were classified into four categories; Operation was direct request of surgical operation, Plan indicates consult about treatment planning and/or strategy of cases, Test was question about concrete testing methods (how to do MR lymphography, etc), and Nutrition was about questions of dose/timing of restart in fasting case. Note the consulters aimed to discuss treatment plans, not simply request for surgical operations. M=months-old, Y=years-old. CoA, coarctation of aorta; HLHS, hypoplastic left heart syndrome; PA, pulmonary artery; PDA, patent ductus arteriosus; PE, pleural effusion; PLE, protein losing enteropathy; TAPVR, total anomalous pulmonary venous return. |
Notably, the major aim of the consultation was the formulation of a treatment plan (23 out of 38, 61%) followed by operative treatment request (19, 50%). Only one consult (out of 38, 3%) was aimed at nutrition advice. Most of our responses to each consultation were in line with the original consultation questions, such as providing an operative treatments, formulating a specific treatment plan, and details of the examination procedure (34 out of 38 consults, 90%). In the remaining four cases (21%), treatment was not actually carried out, since by the time the operation request came there was sudden deterioration of the patient’s general condition, leading to a major shift in the treatment plan (Table 1) .
Physicians who treat chylothorax are facing difficulties in deciding priorities because several novel approaches are available leading to current testing methods and treatment options for central lymphatic disease being updated. In the present study, we aim to organize the consulter types and clinical problems of central lymphatic diseases by analyzing previously received consultations from other hospitals.
As a result, the majorities were in Kanto region, university hospitals, consult from pediatric cardiologists, infantile cases, and aimed specific treatment plans. On the other hand, the types of consultation were similar across regions, hospital types, and specialties. This result indicates that a standardized treatment strategy is necessary for central lymphatic diseases.
Patients with central lymphatic diseases can be diagnosed immediately after as fetal edema,1) but can occasionally occur (6%) posterior to Fontan related cardiac surgery.2) Spontaneous improvement and preservation therapy could be effective; therefore, the treatment plans were decided by the hospitals. However, when it became intractable, treatments could be difficult due to limited therapeutic options, eventually led to prolonged hospitalization and developmental disorders.3)
General treatment strategies for this disease include nutrition therapy (Medium chain triglyceride milk or fasting),4) medical therapy (octreotide or steroids),5) symptomatic surgeries (drainage or thoraco-abdominal shunting), and/or radical surgeries (pleural adhesion or thoracic duct ligation). However, the invasive radical surgeries listed above could be unsuccessful.6, 7)
On the other hand, several novel treatments have been developed based on the concept of normalizing the lymph flow owing to the progression of methods for evaluation of lymph flow.8) Various imaging methods are now in practice to enabled detailed visualization of lymph flow, such as lymphography with inguinal lymph node access.9) The same is also applied to assess deep dynamic lymph flow. Other techniques include dynamic magnetic resonance lymphangiography (MRL),10, 11) to overcome the low resolution of classical lymphoscintigraphy.1) Some reported novel treatments following to those evaluations such as thoracic ductal embolization by interventional radiology (IVR),12) or lymphatic venous anastomosis (LVA) to create bypass for lymphatic flow with microsurgical techniques.13) However, the treatment strategy is not clearly indicated in the previous reports.
From the present study, the importance of common understanding is highlighted, as consults about treatment plans are more popular than actual surgical operation requests. Furthermore, the consulter specialists were mainly physicians, belonging to either cardiovascular, intensive care, or pediatrics specialties, but not surgeons or radiologists who actually perform surgeries. The types of hospitals from where consult request originated were not only specialty hospitals but also general hospitals and university hospitals, where multiple specialists work together. Therefore, we consider it worthwhile to present our strategy for central lymphatic disease, even if it includes personal opinions.
Our Treatment Strategy for Central Lymphatic Disease
We do not insist on the previous classification of central lymph disease, such as congenital, traumatic, or venous congestions. The reasons for this are that diagnosis and treatments are not based on one-to-one correspondence; furthermore, we have also experienced several clinical cases with a combined pathophysiology, as previously reported.14–16) For example, lymphography on postoperative chylothorax after Fontan surgery showed no specific leaking point, instead lymphatic fluid congestion and recurrence in the abdomen or lower, more than just in the thoracic duct. In such cases, the diagnosis should be combined with congenital thoracic obstruction with venous congestion, not a traumatic type of congestion. In short, combined pathophysiology of congenital and venous congestion does exists.
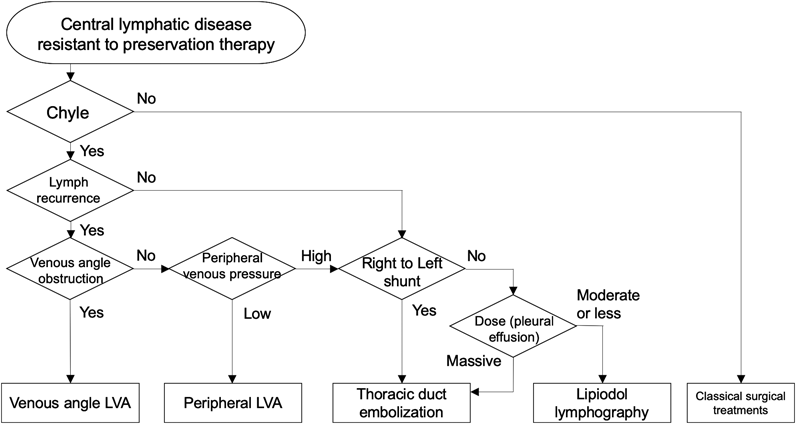
We consider lymphatic flow assessments based on treatment plans as necessary. The current flow-oriented treatments are classified either as “drain”, by bypass creation, or as “plug” by IVR; therefore, we use “drains” for cases with congested lymph, and “plugs” for cases with leakage of lymph. To classify the types of cases, central lymphatic dynamics must be assessed. In other words, when lymphatic congestion or recurrence was confirmed, LVA was used to create a bypass to drain the lymphatic fluid. When orthodromic leakage was visualized, embolization was performed using IVR. Therefore, evaluation of lymph flow recurrence, leakage quantity, and various other points were used as primers to decide the treatment plans.
On the other hand, lymphatic scintigraphy was applied as the first-line technique to visualize the entire flow image. Scintigraphy is advantageous because of its high reproducibility, small differences in results depending on the procedures, and its wide applicability in almost all cases. In addition, fluorescent indocyanine green (ICG) lymphography has been frequently used to evaluate peripheral lymph flow recurrence on the body surface in either the extremities or in the trunk. ICG lymphography follows a very simple procedure: injecting a small amount of agent subcutaneously; therefore, it is highly reproducible. We used ICG lymphography not only for peripheral but also for central lymphatic disease to assess the pathophysiology because it is a less invasive method as compared to other methods; also, there is no risk of radiation exposure, and it can thus be safely used even in neonates. However, observational timing, points to observe, and result evaluation requires experience. Thus, we considered ICG lymphography as a supplemental examination. Although MRL is a valuable method to visualize central lymph flow dynamically and in detail, it is not yet available in many hospitals. For MRL, direct perfusion of the agent into the inguinal lymph node is necessary; therefore, experienced surgeons and radiologic engineers are required. In our experience, owing to the difficulty in puncturing procedures patients younger than 5 years, this technique is not suitable in this population so far. In addition to this, this technique can be quite invasive in small infants. Therefore, we try it whenever possible, but do not consider it as minimal requirement. Savla et al. proposed the classification of central lymph flow with MRL results as traumatic, pulmonary lymphatic perfusion syndrome (PLPS), and central lymphatic flow disorder (CLFD).15) Our proposed combined congestion and recurrence theory was similar to CLFD.
Lymphatic venous anastomosis indicate for obstructive situation at the venous angle (by clot formation), in which all cases showed general lymphatic recurrence. In such cases, cervical lymphatic venous anastomosis is effective at the venous angle, at the exit of the central lymphatic system.13) Peripheral LVA is considered when lymphatic recurrence occurs even without obstruction at the venous angle. In cases with recurrence extending to the extremities, LVA is a good indication because it can be completed less invasively. However, we consider IVR instead, in cases with high peripheral venous pressure, because lymphatic fluid is not easily collected through the bypass created. We suggest LVA for patients older than one week and with body weight greater than 2,500 g.
IVR therapy for central lymphatic disease is represented by thoracic ductal embolization and lipiodol lymphography. Thoracic ductal embolization is highly effective, and an immediate effect is expected; however, special techniques are required, especially in small infants.17) Complications are reported to be limited due to speedy spontaneous collateral formation postoperatively. However, some cases reported intractable iatrogenic lymphedema following lymphatic congestion and recurrence. On the other hand, lipiodol lymphography is a classical evaluation method of lymph flow, and it is expected to be a less invasive therapy. The mechanism of action was considered to be either the agent itself directly obstructing the leakage point due to high viscosity or the local selective adhesion of the pleura following agent leakage.18, 19) However, it is contraindicated in cases with right-to-left shunting because a previous report indicated that lipiodol could cause cerebral infarction through the systemic circulation.20) Therefore, we suggest lipiodol lymphography in cases with relatively small leakage to the thoracic cavity without right to left shunting. It is suitable for patients weighing >1,000 g because microscopic magnification assists in inguinal node injection even in small neonates.
For decision-making, we apply these novel therapies to intractable situations resistant to preservation therapy for at least one month so as to avoid unnecessary surgical interventions, according to previously reported suggestions for surgical interventions.3) Even for intractable chylothorax, we note that pleural effusion assessments are necessary, with property, cell counts, and lymphocyte fraction of effusion. This is because other approaches using a normal lymphatic system could be effective,21) if not chyle (Fig. 4).
The limitations of this study include bias because all the consults were answered by a single team. Consulters were easily contacted if they were acquainted with our team members. Furthermore, consults were mainly from the Kanto region, where our hospital is located. However, we received many similar consults from all over the country, but even these numbers were limited. Furthermore, consults from other specialties were much more popular than ours. Also, concrete treatment strategy is considered to be an important and general concern, as it was the major reason for the consult, especially by pediatric cardiovascular specialists.