Kawasaki disease is an acute self-limiting childhood vasculitis, characterized by hypercytokinemia, which causes valvulitis associated with pancarditis. Valvular heart disease due to valvulitis, such as mitral valve or tricuspid valve regurgitation, is transient and subclinical during the acute phase of Kawasaki disease.1, 2) Although rare, severe valvulitis in the acute or convalescent phase can cause rupture of the mitral chordae tendineae leading to severe mitral valve regurgitation.1, 3) In a nationwide Japanese survey, Tsuda et al. reported that the prevalence of ruptured mitral chordae tendineae due to Kawasaki disease was approximately 1 in 20,000. Six patients with ruptured mitral chordae tendineae were included in that study; three of them required surgical treatment, of which one died.1) Mishima et al. reported a case of Kawasaki disease in a 3-month-old infant who required surgical treatment owing to the progression of heart failure caused by ruptured mitral chordae tendineae.4) These reports indicate that ruptured mitral chordae tendineae associated with Kawasaki disease is a rare, life-threatening complication requiring careful attention, especially in infants.
Herein, we report the case of a 7-month-old girl with incomplete Kawasaki disease who was diagnosed with ruptured mitral chordae tendineae resulting in acute heart failure.
A 7-month-old girl presented with severe mitral regurgitation that led to acute heart failure. Fourteen days before her admission, she had a fever lasting for 6 days. She was taken to a nearby hospital on the third day of illness, although the cause of the fever was unclear, and spontaneous defervescence occurred. According to her parents, she had no other symptoms suggestive of Kawasaki disease during the course of her illness. Food intake and urine output decreased from the 12th day of illness, and she was brought to our hospital on the 14th day.
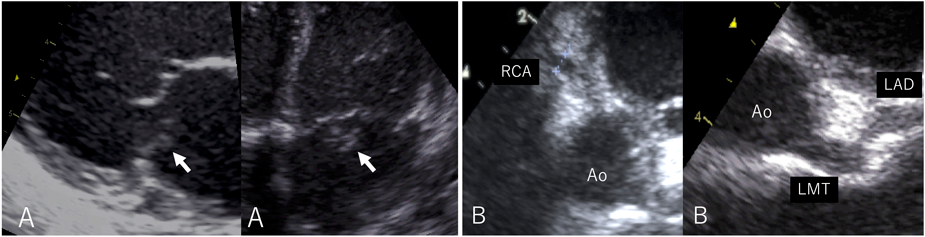
On admission, the body temperature was 36.7°C. A systolic heart murmur, gallop rhythm, and hepatomegaly were confirmed. On the chest radiograph, the cardiothoracic ratio was 50%, and pulmonary congestion was noted. Severe mitral regurgitation due to ruptured chordae tendineae of the posteromedial leaflet was diagnosed using echocardiography (Fig. 1). Aneurysmal dilatation of the proximal coronary artery was also observed. The left main coronary artery was 3.7 mm (z score 5.83), the left anterior descending artery was 3.0 mm (z score 5.30), and the right coronary artery was 3.1 mm (z score 6.13). Laboratory investigations suggested leukocytosis (25,000/µL), thrombocytosis (131×104/µL), hypoalbuminemia (3.1 g/dL), and elevated C-reactive protein level (6.30 mg/dL). Based on the coronary arterial lesion and laboratory data, we diagnosed her with ruptured mitral chordae tendineae due to severe valvulitis resulting from incomplete Kawasaki disease.
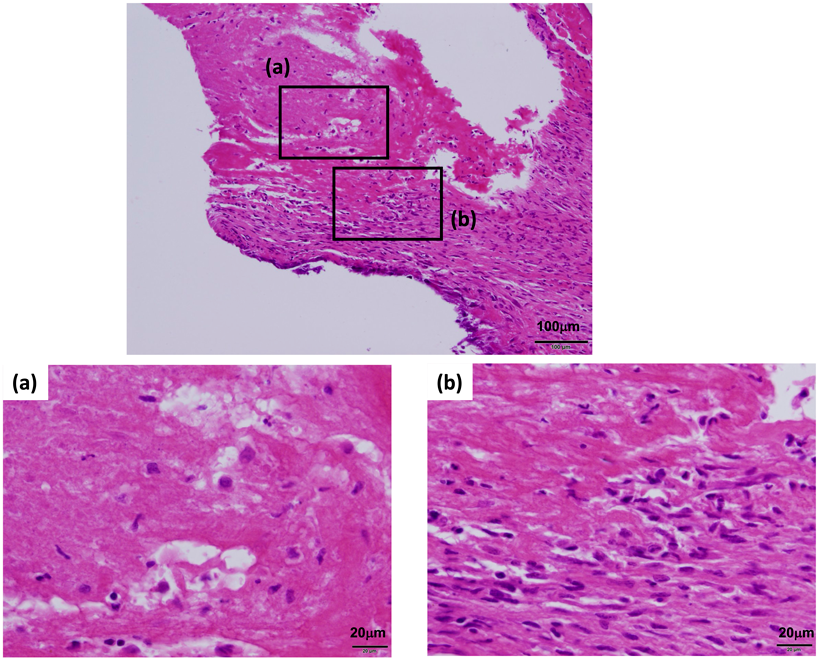
Mechanical ventilation was performed for respiratory failure, intravenous diuretics and milrinone (0.3 µg/kg/min) were started to reduce pulmonary congestion and cardiac afterload. Because of the persistent inflammatory response, she was treated with intravenous immunoglobulin (1 g/kg) and methylprednisolone (30 mg/kg) for 2 days from the day of admission. Despite these treatments, her heart failure progressed. Artificial chordae reconstruction and Kay-Reed annuloplasty were performed on the 17th day of the illness, and on echocardiography, her mitral regurgitation improved from severe to trivial. Intraoperative findings included coronary artery dilatation, yellowish degeneration of the posterior mitral leaflet, and ruptured chordae tendineae. Histopathologic examination of the excised chordae tendineae revealed valvulitis (Fig. 2). The immunohistochemical examination also revealed that most of the inflammatory cells were CD3-positive T lymphocytes.
Oral aspirin (5 mg/kg) was initiated on the 19th day of the illness and continued for 2 months. The postoperative course was favorable, and her clinical symptoms improved.
Desquamation of the fingers was observed on the 39th day of the illness.
Cardiac catheterization, performed on the 49th day of the illness, revealed regression of the coronary aneurysms.
At the age of 5 years, she had a recurrence of typical Kawasaki disease. Aspirin and intravenous immunoglobulin (2 g/kg/day) were administered on the 5th day of the illness, and the fever abated the next day. No cardiovascular complications such as moderate to severe mitral regurgitation or coronary artery dilatation were observed during this recurrence.
Mitral regurgitation is the most common valvular complication caused by Kawasaki disease.1, 2) However, ruptured mitral chordae tendineae in the acute phase of Kawasaki disease is rare. According to Tsuda et al., the rupture of mitral chordae tendineae is likely to occur within 4 months in infants under 6 months old presenting with Kawasaki disease.1) It is considered that the weakening of the chordae tendineae due to inflammation is associated with the younger age.1, 3)
In our case, no valve regurgitation or coronary artery lesions were noted when Kawasaki disease recurred, suggesting that age at onset is a crucial risk factor for ruptured mitral chordae tendineae. The present case is similar to previous reports concerning age and the timing of onset. However, to the best of our knowledge, there have been no previous reports of ruptured mitral chordae tendineae following spontaneous defervescence without the main symptoms of Kawasaki disease, other than fever. The intraoperative and histopathological findings in the present case also showed severe valvular degeneration and persistent inflammation, suggesting that valvulitis in infancy can be severe and independent of clinical symptoms.
The immunohistochemical examination also revealed that most of the inflammatory cells were CD3-positive T lymphocytes. This is similar to the findings in a case of ruptured mitral chordae tendineae in an infant, reported by Shiraishi et al.3) Some cases diagnosed as idiopathic ruptured mitral chordae tendineae in infants may be caused by incomplete Kawasaki disease, as suggested by the present case.
Valvular heart disease in the acute phase of Kawasaki disease is caused by valvulitis due to hypercytokinemia.5, 6) Therefore, it improves with alleviation of the inflammation.
Diagnosis of Kawasaki disease in early childhood is often difficult because of the lack of symptoms.7) Delays in diagnosis may lead to delays in treatment, resulting in a high risk of cardiovascular complications.8) Furthermore, some reports have shown a dissociation between the clinical symptoms and degree of cardiovascular complications. The diagnosis of Kawasaki disease was triggered by the identification of coronary artery lesions after spontaneous defervescence or in the absence of clinical symptoms.8, 9) In this case, coronary artery lesions and laboratory findings also led to the diagnosis of Kawasaki disease; however, reports indicate that ruptured mitral chordae tendineae can occur even in the absence of coronary artery lesions.1) Therefore, we believe it is important to aggressively monitor coronary and valvular disease with echocardiography and consider the possibility of Kawasaki disease when an unexplained fever persists for more than 5 days. Although this case shows that ruptured mitral chordae tendineae can occur even in incomplete Kawasaki disease with early spontaneous defervescence, the accumulation of similar cases will be necessary. This will help further elucidate the mechanisms of valvular disease and identify factors determining its severity, which is important for developing treatment strategies.
In conclusion, patients with mitral regurgitation following valvulitis associated with acute Kawasaki disease, especially in early infancy, should receive frequent evaluation.
Anti-inflammatory treatment should be administered early to avoid life-threatening conditions such as ruptured mitral chordae tendineae.