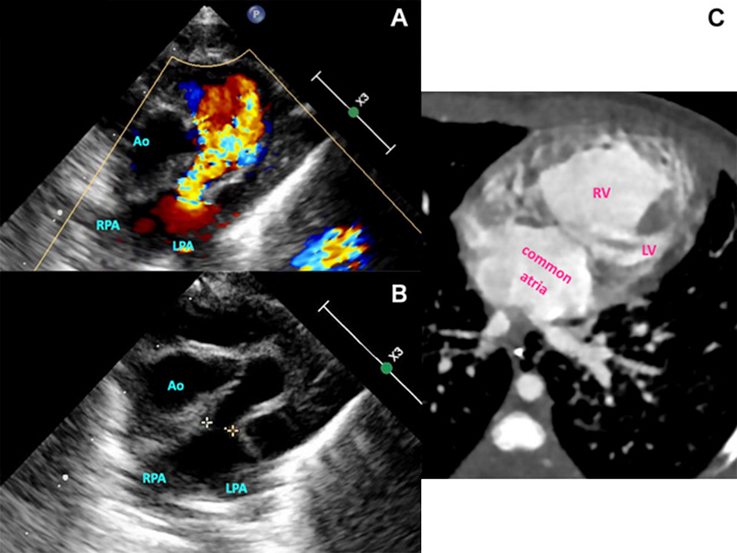
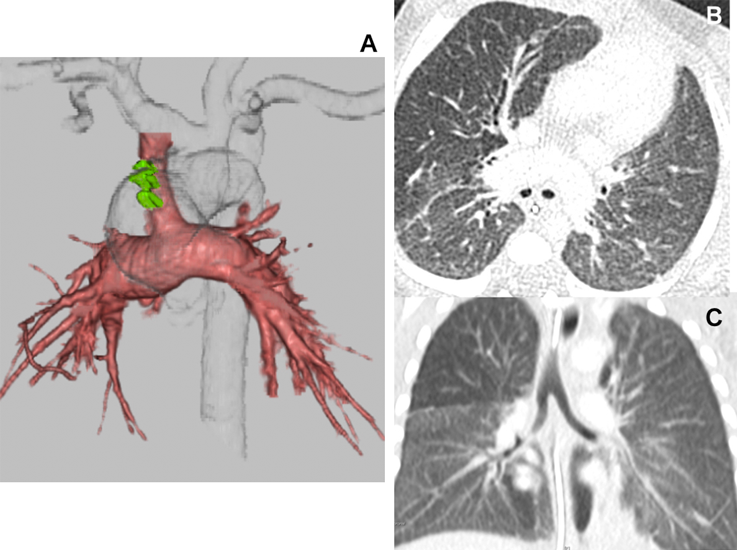
Left Isomerism of the Atrial Appendages with Absent Pulmonary Valve Syndrome and Unbalanced Atrioventricular Septal Defect
Yujiro Ide1,Tadashi Ikeda 1,Shiro Baba2,Takuya Hirata2,Kentaro Akagi2,Kenji Minatoya1Yujiro Ide1, Tadashi Ikeda
1,Shiro Baba2,Takuya Hirata2,Kentaro Akagi2,Kenji Minatoya1Yujiro Ide1, Tadashi Ikeda 1, Shiro Baba2, Takuya Hirata2, Kentaro Akagi2, Kenji Minatoya1
1, Shiro Baba2, Takuya Hirata2, Kentaro Akagi2, Kenji Minatoya1 1 Department of Cardiovascular Surgery, Kyoto University Hospital ◇ Kyoto, Japan
2 Department of Pediatrics, Kyoto University Hospital ◇ Kyoto, Japan
受付日:2021年11月13日Received: November 13, 2021
受理日:2022年6月4日Accepted: June 4, 2022
発行日:2023年3月31日Published: March 31, 2023