Anomalous left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital heart disease with a high natural mortality rate of 90%.1) Dysfunction of the left ventricle (LV) and ischemic mitral regurgitation (MR) are known to be morbid and mortal in this setting. On the other hand, good early and late surgical outcomes have been described following repair of this malformation, thanks to established and standardized approaches for establishing a dual coronary system.2–5) Restoration of the dual coronary system is expected to provide rapid recovery of LV function within the first postoperative year.2, 6)
In patients with poorly formed collaterals between the right and the left coronary arteries, myocardial ischemia can become fatal as pulmonary vascular resistance decreases after birth. In contrast, when communications are extensive between these coronary arteries or when pulmonary hypertension persists due to, for example, congenital heart malformations or impaired lung conditions, these patients may be able to survive beyond infancy without any surgical intervention.2) The latter group, which typically consists of patients undergoing surgery at older age, often presents with normal or near-normal LV size and function.6) Some researchers have claimed that younger operative age may be related to worse morbidity and mortality.5) In reality, little is known about the impact of age at operation on the long-term outcomes and LV function.
The purpose of this study is to review the early and long-term outcomes of ALCAPA repair in our institution, particularly focusing on whether age at operation affects the recovery process of LV function.
We performed a retrospective review of the records of patients who underwent ALCAPA repair at Kanagawa Children’s Medical Center from 2003 to 2018. Patients were divided into two groups based on their age at operation: group Y includes those who underwent ALCAPA repair at the age of 1 year or less, and group O for those over 1 year old. We compared these two groups in terms of the outcomes. This study was approved by the Institutional Review Board of Kanagawa Children’s Medical Center (approval number 2105-8) and carried out according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects. The need for individual or parental consent was waived.
Data Collection
We analyzed in-hospital death rates and the duration of mechanical ventilatory support, intensive care unit (ICU) stay and in-hospital stay for early outcomes; death after discharge and reoperation rate for long-term outcomes; and ejection fraction (EF), fractional shortening (FS), LV end-diastolic dimension (percentage of normal, LVEDD%), MR score, LV end-diastolic volume (percentage of normal, LVEDV%), and LV end-diastolic pressure (LVEDP) for the recovery process of the LV function and dimension. The duration of mechanical ventilatory support was defined as the total time using the mechanical ventilator during hospitalization, irrespective of whether in the ICU or not.
We reviewed the last echocardiographic data recorded prior to repair as the preoperative status and the latest echocardiographic data recorded before discharge as the postoperative circumstance. MR was assessed using Doppler echocardiography and graded as; 0 for no MR, 1 for trivial, 2 for mild, 3 for moderate, and 4 for severe degrees. We performed mitral valve (MV) repair for all patients with MR of grade 3 or higher, regardless of etiology.
Operative Techniques
All patients underwent ALCAPA repair by means of coronary arterial reimplantation. The chest was opened through a median sternotomy. First, we encircled the left main trunk (LMT) of the coronary artery with a delicate snare dissecting its most avascular area. We then occluded the LMT with a small and fine clip to prevent coronary perfusion steal into the pulmonary circulation. This maneuver might improve hemodynamics immediately. It also provides a bloodless operative field without collateral flow through the coronary artery during either harvesting or anastomosing the coronary button. Once the LMT is snared, moreover, the pulmonary arterial trunk can be opened while the heart is beating; the option consequently minimizes aortic cross-clamp time. Cardiac arrest was induced by infusion of cold crystalloid solution into the aortic root. Then, a cannula was selectively inserted into the LMT, and cardioplegic solution was added across the LMT snared. MV repair was carried out in patients with intracardiac anomalies (1 in group Y versus 4 in group O) and in those with moderate or severe MR (4 in group O). The techniques for MV repair included the Kay–Reed annuloplasty, implantation of artificial chordae, and closure of a cleft. The trimmed left coronary arterial button was reimplanted directly onto the aortic root (all except one in group Y) or using the so-called spiral-cuff technique (the remaining one in group Y).7) In all patients, the pulmonary arterial trunk was reconstructed with the autologous pericardium after unclamping the aorta.
Statistical Analysis
We conducted statistical analysis using “EZR” (Saitama Medical Center, Jichi Medical University, Saitama, Japan),8) which is a graphical user interface for “R” (The R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were described as frequencies with percentages. Continuous variables were described as a median with a range or a mean with a standard deviation. Comparisons between two independent groups were calculated using the Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous variables. The Wilcoxon signed-rank test was used to compare two paired groups. To estimate time-related survival and freedom from reoperation after reimplantation, we used the Kaplan–Meier method. Freedom-from-events curves were compared by means of the log-rank test. Statistical significance was set at p<0.05.
Basic Characteristics
Eleven patients (5 in group Y and 6 in group O) underwent ALCAPA repair in our institution. Age at operation was 4.3 (range 1.7–12.1) months in group Y and 94.0 (range 13.3–116.9) months in group O (Table 1), and weight 4.6 (range 4.2–5.8) kg and 17.8 (range 6.8–27.8) kg, respectively. One patient in group Y and 3 in group O had concomitant diagnoses of intracardiac left-to-right shunts. In all these 4, preoperative catheterization showed normal pulmonary arterial pressure. The ratio of pulmonary to systemic blood flow (Qp/Qs) was 1.1 in the patient in group Y. The value was measured over 2.0 in 2 patients in group O. In the remaining one in group O, medical record of the patient indicated pulmonary over-circulation, although no actual figure was documented regarding preoperative Qp/Qs.
Table 1 Basic Characteristics | Group Y (≤1 year old) | Group O (>1 year old) |
|---|
| Total, n | 5 | 6 |
| Gender: male, n (%) | 1 (20) | 2 (33) |
| Age at repair, median (range) [months] | 4.3 (1.7–12.1) | 94.0 (13.3–116.9) |
| Weight at repair, median (range) [kg] | 4.6 (4.2–5.8) | 17.8 (6.8–27.8) |
| Associated diagnoses |
| ASD, n (%) | 0 (0) | 1 (17) |
| VSD alone, n (%) | 1 (20) | 1 (17) |
| VSD with DCRV, n (%) | 0 (0) | 1 (17) |
| Single papillary muscle of LV, n (%) | 0 (0) | 1 (17) |
| Concomitant or past history of mitral valve surgery, n (%) | 1 (20) | 3 (50) |
| ASD, atrial septal defect; DCRV, double-chambered right ventricle; LV, left ventricle; VSD, ventricular septal defect |
MR and MV Surgical Intervention
All patients had MR of grade 1 or severer before surgery (Table 2). The etiologies of MR were exclusively tethering (n=5, 100%) in group Y, whereas prolapse (n=3, 50%), tethering (n=2, 33%), or a cleft (n=1, 17%) in group O. MV repair was concomitantly or had been previously carried out in one patient in group Y and in 3 in group O. Tethering, prolapse, and a cleft were responsible for MR requiring the intervention. One of the three in group O had initially been diagnosed as MR caused by a single papillary muscle before ALCAPA was found, and undergone MV repair using artificial chordae (Table 2). In other patients, closure of a cleft or the Kay–Reed annuloplasty was applied at ALCAPA repair.
Table 2 Difference in MR score changes over time based on their MR etiology| Case | Etiology of MR | Procedures of MVP | MR score | |
|---|
| Preoperative | At discharge | | Final follow-up |
|---|
| 1 | Tethering | — | 2 | 2 | | 0∼1 | Group Y |
| 2 | Tethering | Kay–Reed | 3 | 2 | | 1∼2 |
| 3 | Tethering | — | 2 | 0 | | 0∼1 |
| 4 | Tethering | — | 2 | 2∼3 | | 1 |
| 5 | Tethering | — | 2 | 2 | | 1 |
| 6 | Tethering | — | 1∼2 | 0 | | 1 | Group O |
| 7 | Tethering | Kay–Reed | 4 | 3 | →MVR→ | 0 |
| 8 | Prolapse related to SPM | Artificial chordae | 3∼4 | 2∼3 | | 2∼3 |
| 9 | Prolapse | — | 1 | NA | | 2 |
| 10 | Prolapse | — | 2 | 1 | | 1 |
| 11 | Cleft | Cleft closure | 3 | 3 | | 2 |
| MR score=none (0) ; trivial (1) ; mild (2) ; moderate (3) ; severe (4). NA=Not available. MR, mitral valve regurgitation; MVP, mitral valvuloplasty; MVR, mitral valve replacement; SPM, single papillary muscle |
MR was found less significant at the latest follow-up in the patient in group Y who underwent MV repair. MR in the 3 patients of group O undergoing MV surgery was reduced less adequately. In the seven patients who did not require MV surgery, the degree of MR was grade 2 or lower at the latest follow-up.
Operative Data and Early Postoperative Outcomes
There were no significant differences between the two groups in operation time, cardiopulmonary bypass time, or aortic cross-clamp time (Table 3). No in-hospital death occurred in either of the groups. No significant differences in the rate of delayed sternal closure, use of extracorporeal membrane oxygenation (ECMO), or perioperative complications. One patient required ECMO resuscitation on the ward. The patient left the ICU with mechanical ventilation on the 14th postoperative day, and experienced refractory ventricular arrhythmias on the 35th postoperative day. The event was related to underlying delayed LV recovery and respiratory overload during weaning from a mechanical ventilator. Keeping the heart condition under control on inotropes and antiarrhythmic agents, the patient could be weaned off ECMO within 24 hours.
Table 3 Intraoperative and postoperative outcomes | Group Y | Group O | p-Value |
|---|
| Intraoperative outcomes |
| Operation time, mean±SD [min] | 319±160 | 258±60 | 0.54 |
| CPB time, mean±SD [min] | 195±112 | 134±26 | 0.25 |
| AoX time, mean±SD [min] | 79±67 | 61±38 | 0.79 |
| Postoperative outcomes |
| Delayed sternal closure, n (%) | 2 (40) | 0 (0) | 0.18 |
| ECMO insertion, n (%) | 1 (20) | 0 (0) | 0.46 |
| Operative or postoperative complications | | | |
| Pulmonary artery branch stenosis, n (%) | 1 (20) | 0 (0) | 0.46 |
| Diaphragmatic nerve palsy, n (%) | 0 (0) | 1 (17) | 1 |
| Arrhythmias, n (%) | 2 (40) | 1 (17) | 0.55 |
| Inhospital mortality, n (%) | 0 (0) | 0 (0) | 1 |
| Mechanical ventilation, median (range) [days] | 6 (2–140) | 1 (1–2) | 0.005 |
| ICU length of stay, median (range) [days] | 6 (3–29) | 2 (2–4) | 0.01 |
| Hospital length of stay, median (range) [days] | 17 (12–202) | 6 (5–12) | 0.007 |
| Long-term outcomes |
| Follow-up time, median (range) [years] | 14.6 (2.0–16.2) | 11.1 (1.8–16.4) | 0.93 |
| Deaths at follow-up, n (%) | 0 (0) | 0 (0) | 1 |
| Reoperation at follow-up, n (%) | 0 (0) | 1 (17) | 1 |
| Mitral valve replacement, n (%) | 0 (0) | 1 (17) | 1 |
| Postoperative cardiac catheterization |
| Patency of reimplanted LCA, n (%) | 4 (80) | 6 (100) | 0.46 |
| LVEF, median (range) [%] | 74.3 (58.0–75.0) | 66.5 (53.0–72.0) | 0.45 |
| LVEDV (% of normal), median (range) [%] | 139 (121–188) | 93 (76–142) | 0.03 |
| LVEDP, median (range) [mmHg] | 10 (4–12) | 9.5 (7–12) | 0.91 |
| Follow-up time of catheterization, median (range) [years] | 2.0 (0.5–5.5) | 1.6 (0.9–15.2) | 0.83 |
| AoX, aortic cross-clamp; CPB, cardiopulmonary bypass time; ECMO, extracorporeal membrane oxygenation; LVEDP, left ventricular end-diastolic pressure; LVEDV, left ventricular end-diastolic volume; SD, standard deviation |
Duration of mechanical ventilatory support was significantly longer in group Y (a median 6 days (range 2–140) in group Y versus 1 day (range 1–2) in group O; p<0.01). Also, duration of ICU stay (a median 6 days (range 3–29) in group Y versus 2 days (range 2–4) in group O; p=0.01), and in-hospital stay (a median 17 days (range 12–202) versus 6 days (range 5–12) in group O; p<0.01).
Echocardiographic Data
Table 4 presents echocardiographic data of each age group in a time series way. Overall, the interval between ALCAPA repair and the latest echocardiographic follow-up was 12.7 (range 1.6–15.6) years. Over the period of time, EF of the LV improved from 44.8% (22.2–72.6%) to 74.7% (59.0–84.1%) (p<0.01), FS from 0.18 (0.08–0.37) to 0.40 (0.31–0.46) (p<0.01), LVEDD% from 160% (104–223%) to 125% (82–212%) (p<0.01), and MR score from 2 (1–4) to 1 (0–2∼3) (p=0.02).
Table 4 Echocardiographic findings | Overall | Group Y | Group O | p-Value |
|---|
| Preoperative |
| EF [%] | 44.8 (22.2–72.6) | 32.2 (22.2–38.7) | 60.0 (44.8–72.6) | 0.004 |
| FS | 0.18 (0.08–0.37) | 0.15 (0.08–0.18) | 0.31 (0.18–0.37) | 0.01 |
| LVEDD (% of normal) [%] | 160 (104–223) | 187 (137–223) | 119 (104–170) | 0.052 |
| MR score | 2 (1–4) | 2 (2–3) | 2∼3 (1–4) | 0.77 |
| At discharge |
| EF [%] | 56.0 (10.3–78.6) | 36.0 (10.3–77.1) | 66.0 (47.0–78.6) | 0.15 |
| FS | 0.31 (0.13–0.46) | 0.20 (0.13–0.39) | 0.34 (0.19–0.46) | 0.55 |
| LVEDD (% of normal) [%] | 125 (82–212) | 125 (92–212) | 125 (82–144) | 0.84 |
| MR score | 2 (0–3) | 2 (0–2∼3) | 2 (0–3) | 0.46 |
| Mid-term follow-up |
| EF [%] | 65.0 (17.1–77.1) | 64.0 (17.1–77.1) | 67.1 (24.0–72.6) | 0.56 |
| FS | 0.32 (0.06–0.39) | 0.32 (0.06–0.39) | 0.31 (0.09–0.35) | 1 |
| LVEDD (% of normal) [%] | 114 (91–227) | 116 (92–227) | 111 (91–145) | 0.53 |
| MR score | 1 (0–3∼4) | 1 (0–3) | 2∼3 (0–3∼4) | 0.75 |
| Follow-up time of echocardiography [months] | 5.1 (2.1–19.4) | 5.8 (2.1–10.0) | 4.4 (2.4–19.4) | 0.92 |
| Final follow-up |
| EF [%] | 74.7 (59.0–84.1) | 73.2 (59.0–75.3) | 75.7 (68.6–84.1) | 0.13 |
| FS | 0.40 (0.31–0.46) | 0.37 (0.31–0.42) | 0.43 (0.32–0.46) | 0.14 |
| LVEDD (% of normal) [%] | 105 (84–129) | 115 (95–129) | 104 (84–107) | 0.25 |
| MR score | 1 (0–2∼3) | 1 (0∼1–1∼2) | 1∼2 (0–2∼3) | 0.26 |
| Follow-up time of echocardiography [years] | 12.7 (1.6–15.6) | 12.7 (2.0–15.1) | 9.2 (1.6–15.6) | 0.79 |
| Values are median (range). MR score=none (0); trivial (1); mild (2); moderate (3); severe (4). EF, ejection fraction; FS, fraction shortening; LVEDD=left ventriclular end-diastolic dimension; MR, mitral valve regurgitation |
The preoperative EF and FS were significantly lower in group Y, and preoperative LVEDD% tended to be higher. LVEDD% in this group became almost normal by the time of discharge, whereas EF and FS appeared to remain lower than those in group O, although detecting no statistical difference. EF and FS in group Y significantly improved at a median of 5.8 months (range 2.1–10.0) after ALCAPA repair.
The interval between ALCAPA repair and the latest echocardiographic follow-up was 12.7 (range 2.0–15.1) years in group Y and 9.2 (range 1.6–15.6) years in group O. There were no significant differences in EF, FS, LVEDD%, or MR score between the two groups after the mid-term follow-up.
Long-Term Outcomes
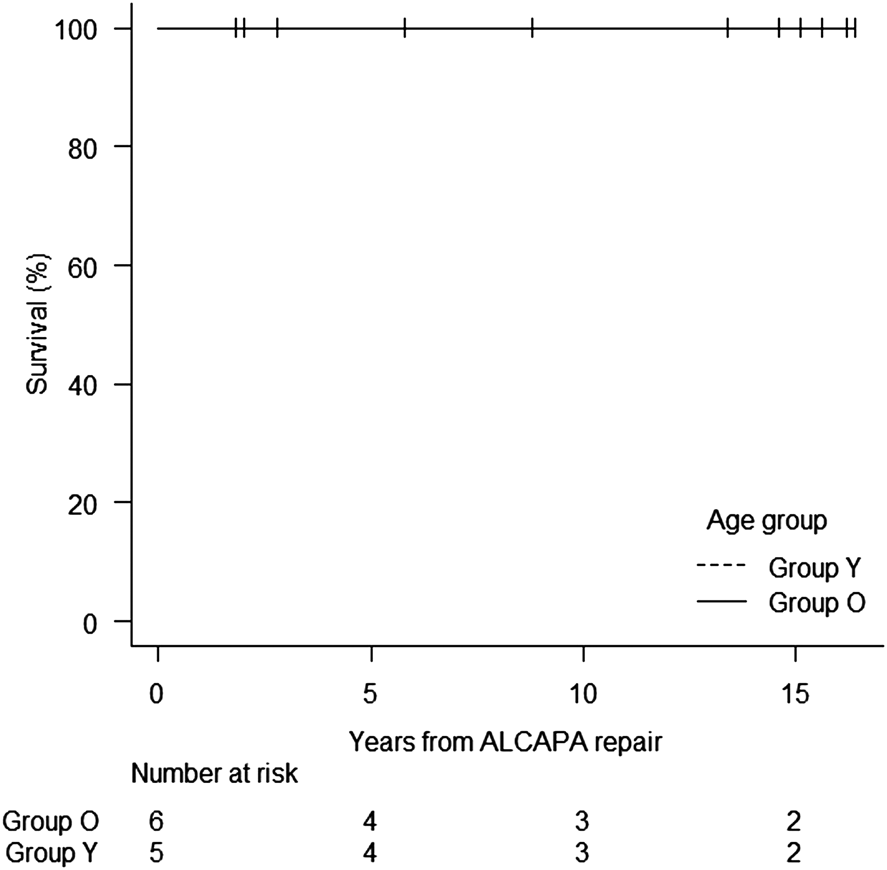
There were no late deaths in the follow-up of 14.6 (range 2.0–16.2) years in group Y and 11.1 (range 1.8–16.4) years in group O (Table 3). Kaplan–Meier survival rate was 100% at 5, 10, and 15 years in both groups (Fig. 1).
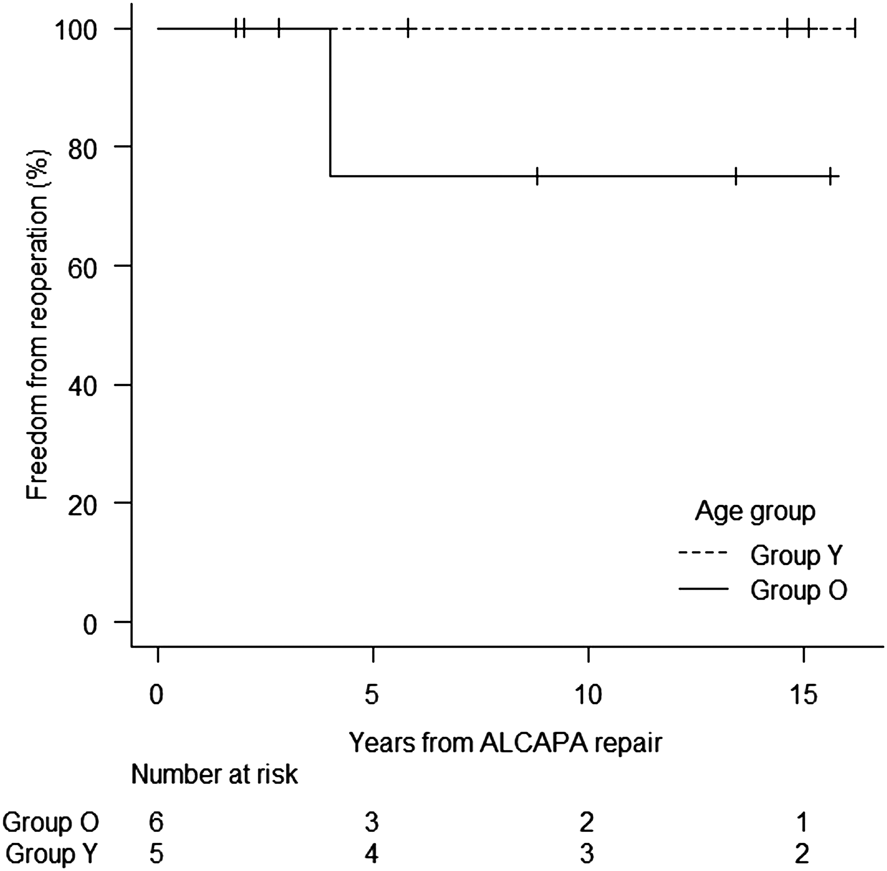
Kaplan–Meier freedom from reoperation was 100% at 1, 5, 10, and 15 years in group Y and 100% at 1 year and 75% (95% confidence interval, 13–96%) at 5, 10, and 15 years in group O (Fig. 2). One patient required reoperation for MV 4 years after ALCAPA repair. The patient had concomitantly undergone MV repair for MR grade 4 at ALCAPA repair, with MR grade 2 at discharge. Subsequently, MR got worse (MR grade 3) and LVEDD% increased to 209%. MV was therefore replaced.
LVEDV% measured from the latest follow-up cardiac catheterization was greater in group Y (139% (range 121–188%) versus 93% (range 76–142%) in group O; p=0.03). LVEDP was not different between the two groups; relatively high in both groups. Cardiac catheterization illustrated that the reimplanted left coronary artery was patent in 80% of group Y and in 100% of group O (p=0.46).
This study demonstrates that ALCAPA repair by coronary reimplantation could provide good outcomes overall. The duration of mechanical ventilatory support, ICU stay, and in-hospital stay were all significantly longer in group Y. The LV appeared to take a few months longer in group Y than in group O before reaching a fair level of function and dimension, although not statistically significant.
Early Postoperative Outcomes
Given that the published early mortality after ALCAPA repair ranges from 0% to 16%,2, 4, 9) the early postoperative outcomes in our study were good. Since there was no early mortality in our cohort, it was not feasible to analyze the risk factors for in-hospital deaths. A report described younger age at operation, preoperative episode of myocardial infarction, and preoperatively low FS or severe MR as unfavorable predictors.9)
With regard to why infants require longer duration of mechanical ventilatory support, ICU stay, and in-hospital stay, we speculate that preoperatively lower EF and FS reflected severer heart failure in group Y and thus recovery was delayed in the acute phase. Azakie et al. and Caspi et al. also reported that younger age at operation was related to longer stay in the ICU,5, 9) which was compatible with our results. On the other hand, Weigand et al. pointed out a potential bias that a clinician tended to decrease ventilatory and inotropic supports more slowly than usual in those with preoperatively severe LV dysfunction. In this respect, we should be careful how to interpret these results.
Recovery of LV Function
Overall LV function indicated by EF, FS, LVEDD%, and MR score became normal by the latest echocardiographic follow-up. Similar findings have been reported by many previous studies.2–6, 9)
EF and FS came up gradually in group Y, and the median values for these parameters caught up with those in group O after 5.8 months, although no statistical difference detected. This trend of relatively late recovery is probably, again, related to preoperatively lower EF and FS in group Y. We speculate that preoperatively less impaired LV function in some patients in group O was partially because of an intracardiac left-to-right shunt of Qp/Qs over 2 which raises pulmonary arterial oxygen saturation and consequently oxygen supply to the left coronary artery. Normalization of EF reportedly occurs by 3.5 months.5) The finding is consistent with our results.
There was no significant difference in the recovery courses of LV function and dimension after the mid-term follow-up between the two groups. Information regarding the influence of patient age at ALCAPA repair on improvement in LV function seems limited in the literature. Lange et al. concluded that LV function significantly improved, regardless of age at ALCAPA repair, in their study focusing on 78 patients who underwent ALCAPA repair with a median follow-up period of 15 years.2) The mechanisms for their finding that recovery of LV function was age-independent remain uncertain. Some researchers have suggested that the ability of the cardiomyocytes for turnover or regeneration could decline with age.10, 11) We assume that the reason our two groups showed no difference in LV function in the longer term was reversible hypoperfusion or hypoxia of the cardiomyocytes, rather than a definite loss of the tissue; the preoperative status appeared worse in group Y because of less adequate oxygen delivery to the myocytes as the major mechanism of reduced LV function.2)
Many previous studies used such traditional parameters as EF, FS, and LV dimensions to evaluate LV function, and documented excellent LV recovery after ALCAPA repair. A recent study, however, demonstrated the prolonged impairment of LV function and residual myocardial damage, even in patients undergoing successful ALCAPA repair, which might be difficult to detect using traditional measurements.12) Some articles proposed use of detecting diastolic dysfunction of the LV, a decrease in global longitudinal strain on speckle tracking echocardiography, and focal perfusion deficit due to scars of the LV myocardium on cardiac magnetic resonance imaging.12–14) In our study, cardiac catheterization showed higher LVEDV in group Y, whereas the mid-term echocardiographic data did not show difference in LVEDD% between the two groups. It is hard to conclude clearly on the basis of these different types of LV dimension variables. Multifaceted approaches will be needed to evaluate and to understand long-term LV function still further.
Strategy for MR
How to manage MR at the time of ALCAPA repair remains controversial. Some recommend annuloplasty for all patients with severe MR.13, 15) Others emphasize etiology rather than severity of MR; suggesting MV repair only when MR was caused by structural lesions.16) Caspi et al. concluded that MR is to be treated at ALCAPA repair in older children and adolescents, because the impediment in these populations, unlike during infancy, is usually caused by irreversible myocardial damage or papillary muscle infarction.9) Naimo et al. reported that they did not perform MV repair concomitantly in 6 of 11 patients who had severe MR; of the 6 patients, 3 eventually required MV repair or replacement during the follow-up period.4) This probably indicates that concomitant MV repair would have been sensible at ALCAPA repair in those with severe MR to minimize late MV reinterventions. On the other hand, some authors cautioned against a potential of increased operative risks due to the addition surgical maneuver which should prolong aortic cross-clamp time.17)
Our strategy has been for carrying out concomitant MV repair in all patients with MR grade 3 or higher. Among the 4 patients who underwent concomitant MV surgery in our cohort, MR improved less drastically in 3 (group O) at the latest follow-up. Congenital structural factors such as a single papillary muscle and a clefted MV could have made MR regulation more difficult. The 3 patients in group O had residual MR of a higher grade at discharge, and MR did not get obviously better during the longer term follow-up. Another theoretical explanation for difficulty in MR regulation is that myocardial damage and infarction of the papillary muscles could be irreversible in older children, as Caspi et al. described.9) If so, simple annuloplasty such as the Kay–Reed procedure is unlikely sufficient. When the leaflets do prolapse because of elongated chordae tendinea and dysfunction of the papillary muscles, MV repair using artificial chordae should be a technique of choice.
The optimal strategy for MR combined with ALCAPA has yet to be determined; this condition remains relatively uncommon. Our current series of patients was small in number, and unable to show concrete statistical data on MR. Still, we believe that analyzing individual mechanism of MR is important. The classification of MR etiology used in adults might be helpful.18)
Limitations
This study is a single-center and retrospective review conducted with a limited number of patients. Because of the wide span of examination period for echocardiography and cardiac catheterization, it was difficult to precisely evaluate and compare the process of LV function recovery. In addition, we could not exclude the possibility that figures measured on these examinations had been influenced by difference levels of preoperative inotropic support. We were unable to obtain qualitative data how much collaterals had developed between the right and the left coronary arteries; therefore, we could not evaluate the impact of such collateral flows. The statistical comparison was limited because of a relatively small number of patients. Univariable analysis was only the way, and accordingly many confounding factors might underlie.
Establishment of a dual coronary system by reimplantation of the left coronary artery to the aorta for ALCAPA provided excellent early and late survival with a low reintervention rate, regardless of age at repair. LV function and dimension in the younger group tended to take a few months longer before catching up with those in the older group, although not statistically different. Lifelong surveillance is required for further discussion not only by standard echocardiographic evaluation but also by multifaceted approaches including speckle tracking echocardiography and magnetic resonance imaging.
Conflicts of Interest
No authors declare conflict of interest.
引用文献References
1) Wesselhoeft H, Fawcett JS, Johnson AL: Anomalous origin of the left coronary artery from the pulmonary trunk: Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation 1968; 38: 403–425
2) Lange R, Cleuziou J, Krane M, et al: Long-term outcome after anomalous left coronary artery from the pulmonary artery repair: A 40-year single-centre experience. Eur J Cardiothorac Surg 2018; 53: 732–739
3) Lange R, Vogt M, Hörer J, et al: Long-term results of repair of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg 2007; 83: 1463–1471
4) Naimo PS, Fricke TA, d’Udekem Y, et al: Surgical intervention for anomalous origin of left coronary artery from the pulmonary artery in children: A long-term follow-up. Ann Thorac Surg 2016; 101: 1842–1848
5) Azakie A, Russell JL, Mccrindle BW, et al: Anatomic repair of anomalous left coronary artery from the pulmonary artery by aortic reimplantation: Early survival, patterns of ventricular recovery and late outcome. Ann Thorac Surg 2003; 75: 1535–1541
6) Weigand J, Marshall CD, Bacha EA, et al: Repair of anomalous left coronary artery from the pulmonary artery in the modern era: Preoperative predictors of immediate postoperative outcomes and long term cardiac follow-up. Pediatr Cardiol 2015; 36: 489–497
7) Ando Y, Kado H, Masuda M, et al: “Spiral-cuff” technique for repair of anomalous left coronary artery from the pulmonary artery. Ann Thorac Surg 2008; 86: 667–668
8) Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458
9) Caspi J, Pettitt TW, Sperrazza C, et al: Reimplantation of anomalous left coronary artery from the pulmonary artery without mitral valve repair. Ann Thorac Surg 2007; 84: 619–623
10) Porrello ER, Mahmoud AI, Simpson E, et al: Transient regenerative potential of the neonatal mouse heart. Science 2011; 331: 1078–1080
11) Bergmann O, Bhardwaj RD, Bernard S, et al: Evidence for cardiomyocyte renewal in humans. Science 2009; 324: 98–102
12) Dąbrowska-Kugacka A, Dorniak K, Meyer-Szary J, et al: Myocardial function in patients with anomalous left coronary artery from the pulmonary artery syndrome: A long-term speckle tracking echocardiographic study. PLoS One 2019; 14: e0223227
13) Alexi-Meskishvili V, Nasseri BA, Nordmeyer S, et al: Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. J Thorac Cardiovasc Surg 2011; 142: 868–874
14) Cabrera AG, Chen DW, Pignatelli RH, et al: Outcomes of anomalous left coronary artery from pulmonary artery repair: Beyond normal function. Ann Thorac Surg 2015; 99: 1342–1347
15) Isomatsu Y, Imai Y, Shin’oka T, et al: Surgical intervention for anomalous origin of the left coronary artery from the pulmonary artery: The Tokyo experience. J Thorac Cardiovasc Surg 2001; 121: 792–797
16) Kudumula V, Mehta C, Stumper O, et al: Twenty-year outcome of anomalous origin of left coronary artery from pulmonary artery: Management of mitral regurgitation. Ann Thorac Surg 2014; 97: 938–944
17) Huddleston CB, Balzer DT, Mendeloff EN: Repair of anomalous left main coronary artery arising from the pulmonary artery in infants: Long-term impact on the mitral valve. Ann Thorac Surg 2001; 71: 1985–1988
18) El Sabbagh AE, Reddy YNV, Nishimura RA: Mitral valve regurgitation in the contemporary era: Insights into diagnosis, management, and future directions. JACC Cardiovasc Imaging 2018; 11: 628–643