A 10-year-old boy presented with the chief complaint of pain in the right foot, with localized redness, swelling, and heat. He had a history of SVAS which was diagnosed at 2 months of age; as his mother also had SVAS and had undergone surgery, familial SVAS was suspected. At 2 years of age, a cardiac catheter examination revealed a pressure gradient of 43 mmHg between the proximal and the distal ascending aorta, prompting surgery to relieve aortic narrowing using the Myers technique without artificial materials. After surgery, echocardiography showed a gradual narrowing of the SVAS. Eight years after the operation, the estimated pressure gradient came up to 48 mmHg across the SVAS on transthoracic echocardiography. Consequently, he was scheduled for cardiac catheterization to determine whether he should have a surgical revision. Unfortunately this patient got ill with IE before the scheduled admission. Three months prior to admission due to IE, he complained of pain in his upper and lower extremities without fever, visiting another orthopedic clinic several times. The cause of the pain was not identified. No further investigation, no antibiotics. Four days prior to admission, he lost his baby teeth naturally.
On the day of admission to our hospital, his physical findings were as follows; body temperature 38.0°C, heart rate 91 bpm, brachial blood pressure 129/64 mmHg, respiratory rate 20/min, and SpO2 97% (room air). He had a grade 2/6 systolic murmur at the second intercostal space on the right sternal border. He experienced swelling, redness, heat, and pain in the right leg. The patient had no hepatomegaly or splenomegaly. No petechiae in the palpebral conjunctivae or oral mucosa. No Janeway lesions, no Osler nodes on the fingers or the toes. No splinter hemorrhages on the nail bed. Further, the patient had no decayed teeth. Blood test results showed; white blood cells 8,400/µL, neutrophils 81.6%, C-reactive protein 6.86 mg/dL, and rheumatoid factor 34 IU/mL (normal value 0–15 IU/mL). Tests for cyclic citrullinated peptide antibody and antinuclear antibody were negative.
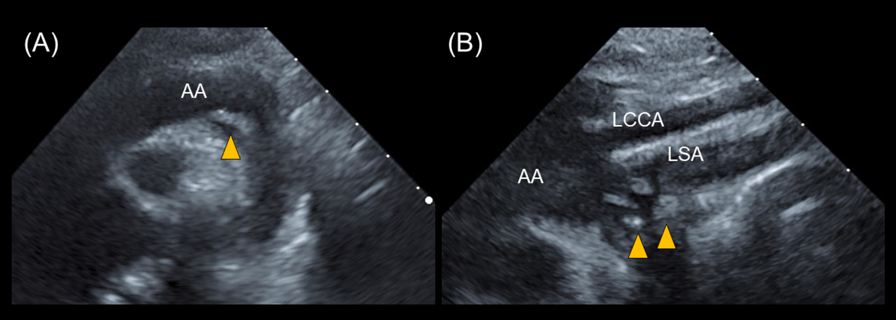
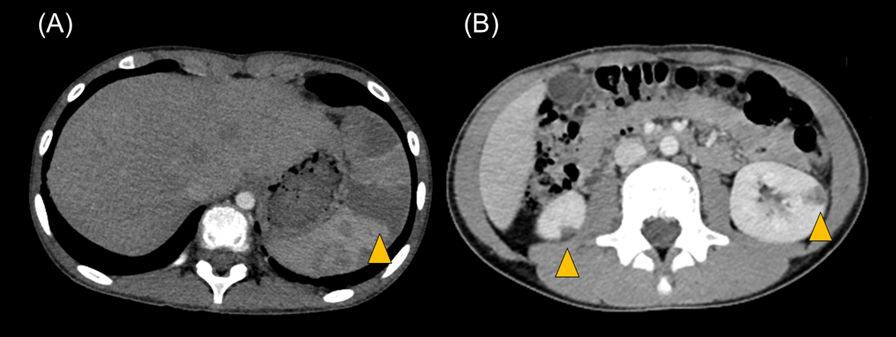
Transthoracic echocardiography revealed multiple vegetations in the aortic arch (Fig. 1). Thoracic contrast-enhanced CT indicated not only SVAS but also stenosis at the origin of either the brachiocephalic artery or the left common carotid artery (Fig. 2). At the time of admission, the patient also experienced left-sided abdominal pain and nausea. Abdominal contrast-enhanced CT revealed renal and splenic infarctions without any arterial aneurysm (Fig. 3). Magnetic resonance imaging (MRI) revealed a very small brain hemorrhage scar. Blood culture detected Abiotrophia defectiva, an endemic oral bacterium that shows penicillin sensitivity. We made a diagnosis of IE according to the modified Duke criteria.4) Urgent surgery for removing vegetations was performed to avoid cerebral embolisms, followed by antibiotic therapy using both penicillin 300,000 U/kg/day and Gentamycin 3 mg/kg/day.
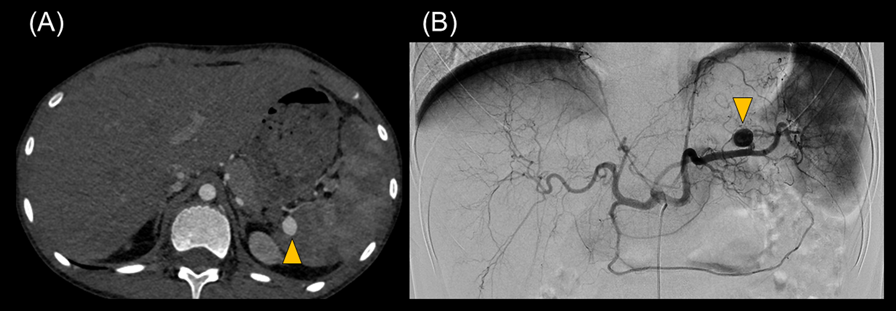
One month after the operation, we repeated the abdominal contrast-enhanced CT scan to follow up renal and splenic infarctions. At this time, we unexpectedly detected a splenic artery aneurysm that was 10 mm in size. Semi-urgent percutaneous coil embolization was carried out using a catheter to avoid aneurysm rupture (Fig. 4). Radiologists approached the SAA using microcatheters, Sniper 2 high-flow type (Terumo, Tokyo, Japan), and MARVEL (Tokai Medical Products, Kasugai, Japan) through a C2 catheter of 4-French (Medikit, Tokyo, Japan) at his celiac trunk. The distal and the proximal portions of the aneurysm were occluded by several coils using Target XL (Stryker Neurovascular, Fremont, CA, US) and C-stopper of 0.014 inch (PIOLAX, Kanagawa, Japan) (Fig. 5). No aneurysm rupture occurred, nor recurrence of IE after embolization.
Two months after completion of the 7-week antibiotic treatment, an aortic replacement surgery was performed to relieve the aortic stenosis. Pathological examination of the SVAS tissue revealed neointimal formation, necrosis of the smooth muscle in the media, and no inflammatory cells (Fig. 6). Elastic or collagenous abnormalities, as seen in Williams–Beuren, Ehlers–Danlos, or Marfan syndrome, were not observed. Regarding familial SVAS, we performed fluorescence in situ hybridization for Williams syndrome, microarray chromosome analysis by comparative genomic hybridization, and whole-exome sequencing analysis. No pathogenic findings were identified in any of these tests, and the genetic cause remained unknown.
SVAS is a congenital heart disease caused by narrowing of the ascending aortic lumen and generation of a turbulent jet flow toward the aortic arch, which can lead to endothelial dysfunction, followed by nonbacterial or bacterial thrombosis and/or vegetation at the aortic arch.5) Finding a vegetation related to SVAS can be difficult, since the aortic arch is difficult to illustrate on transthoracic echocardiography. In the present case, it took a fairly long time before identifying IE, admitting that the patient had been afebrile until he visited our hospital. The symptom of pain in his extremities could have been caused by very small embolisms of the extremities generated by tearing off of a thrombus at the central arterial pathway. Consistent with this, his abdominal contrast-enhanced CT showed renal and splenic infarctions, and his head MRI indicated very small brain hemorrhage scars. It is difficult to determine the real onset of IE in this patient. We speculate that SVAS caused a nonbacterial thrombus on the endothelium in his aortic arch, and that the embolisms of his extremities from nonbacterial thrombosis led to afebrile pain in the extremities. A wobbly tooth could have caused bacteremia and generated bacterial vegetation on the thrombus. We believe that this patient should have been on antithrombotic therapy, such as aspirin, to prevent thrombosis due to turbulent jet flow caused by SVAS.
Abiotrophia defectiva is a part of the normal flora in the oral cavity. It is a pleomorphic organism that appears as gram-positive cocci and bacilli, depending on the culture media. Although it causes less than 1% of IE cases, Abiotrophia defectiva has a high probability of producing embolic complications and valve destruction. Abiotrophia defectiva has a higher affinity for the endocardium and has the ability to bind fibronectin in the extracellular matrix. Most known cases of IE associated with the organism are associated with vegetation sizes >10 mm. Indeed, approximately 50% of the patients require surgical management, despite sensitivity of Abiotrophia defectiva to antibiotics.6)
The most important learning point in this case is that IE can cause the infected SAA. Splenic abscesses and infarctions are not uncommon as complications of IE, while an infected SAA is rare, especially in children.7) Case reports regarding SAA in childhood are limited, with etiologies including trauma, acute pancreatitis,8) Marfan’s syndrome,9) and Menkes disease.10) Interestingly, a few papers describe that Abiotrophia defectiva induces not only infarction by vegetations but also aneurysm in the brain and mesenteric arterial branch in childhood.11, 12) Some clinical cases in adults have demonstrated that an infected SAA can expand more rapidly in size and subsequently rupture more often as compared to atherosclerotic aneurysms.7) Even though infected SAAs are rare, their potential to rupture represents a serious complication of IE.13) Infected SAAs in adults is frequently symptomatic and mostly present with symptoms of abdominal pain and bleeding from the gastrointestinal tract.2) We speculate that the infected SAA in our case was developing or getting greater during several weeks of antibiotic therapy between the first and the second contrast-enhanced CT investigations. We considered that Abiotrophia defectiva could have been still alive during the period and that this case should have required penicillin therapy for a longer term, despite the recommendation by the American Heart Association ampicillin or penicillin G in addition to gentamicin for a period of 4–6 weeks.14)
Urgent intervention for SAA is recommended when; (1) symptomatic, (2) size >2 cm, and (3) documented enlargement.3) CT angiography is a recommended imaging modality to investigate infected aneurysm. Further, arteriography is the gold standard for diagnosis as well as for clarifying localization of the lesion, eventually allowing percutaneous vascular intervention.3) Treatment options include administration of antibiotics, transcatheter embolization, and surgical resection. There is a risk of residual infection in the SAA after transcatheter embolization even though the technique turns out successful in preventing SAA rupture. We selected to occlude the distal and the proximal portions of the aneurysm. We considered that collateral arteries would feed the spleen. This strategy avoids a residue of the aneurysm supplied by collateral arteries, and prevents recurrence due to coil compaction or sac growth after packing coils in the aneurysm. Surgical resection of the aneurysm with vascular reconstruction is a suitable approach for large SAAs once embolization failed.2)
We successfully coil-embolized a 10 mm SAA on a semi-urgent basis, without rupture of the aneurysm or recurrence of IE after embolization. The reasons why this patient developed SAA seem multifactorial. We speculate that familial arteriopathy, including SVAS and stenoses at the brachiocephalic and the left common carotid arteries, is associated with aneurysmal formation related to IE, although we could not find a causative gene such as elastin in this case. As for Abiotrophia defectiva, we recommend repeated imaging evaluations even if the patient remains asymptomatic, given the higher risk of adverse events such as infarctions and aneurysms.