A 3-day-old female neonate (39 weeks gestation and 2,378 g birth weight) was referred to our institution due to LV dysfunction. Her Apgar score was 8 at 1 min and 8 at 5 min. She was initially admitted to a neonatal intensive care unit for a postnatal respiratory disorder, and echocardiography revealed LV dysfunction. Blood tests showed high levels of creatine kinase (CK: 1,056 IU/L) and lactate dehydrogenase (LDH: 1,221 IU/L), indicating intense stress during delivery. Respiratory status was stabilized with a high-flow nasal cannula, while LV dysfunction did not improve. She was eventually transferred to our hospital for further examination of her cardiac dysfunction.
On arrival, her vital signs were stable (heart rate 150 beats/min, blood pressure 63/44 mmHg, respiratory rate 45 breaths/min, temperature 37.1°C, oxygen saturation 98%). Chest radiography showed cardiac enlargement with a cardiothoracic ratio of 57%. The electrocardiogram showed sinus rhythm with a pattern of right ventricular hypertrophy. Echocardiography illustrated no congenital structural malformation or coronary arterial abnormality.
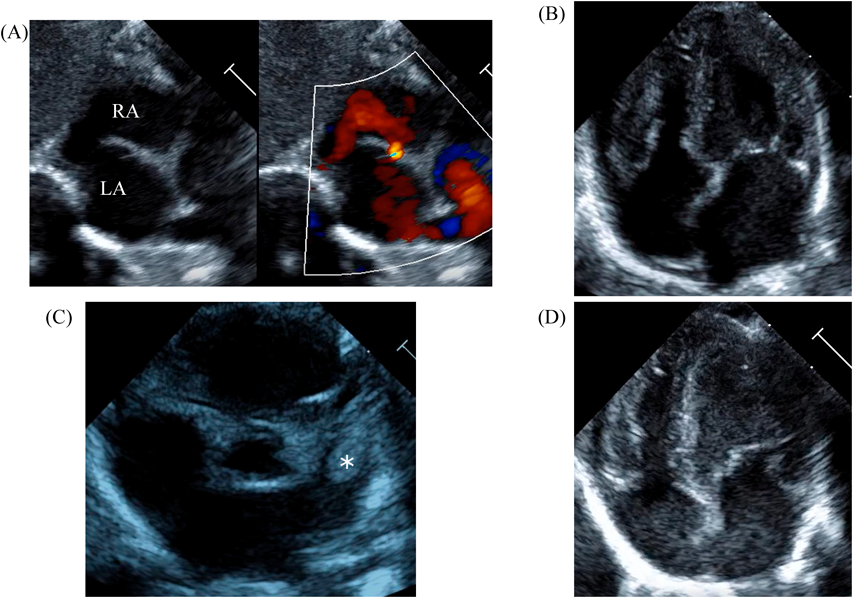
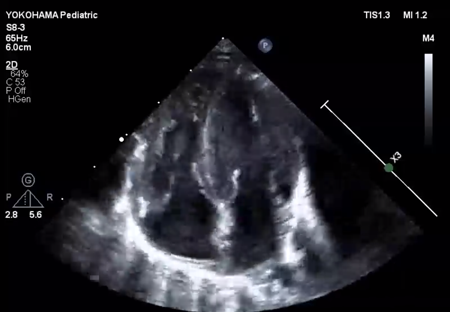
LV ejection fraction was down to 40% with moderate mitral regurgitation, whereas RV contractility was preserved. The biventricular volumes seemed well-balanced, and the LV end-diastolic diameter (15.9 mm) was within the normal range. Systolic RV pressure was estimated as 70 mmHg by regurgitation flow across the tricuspid valve (velocity 4.1 m/s), indicating persistent pulmonary hypertension. The ductus arteriosus had already closed. The foramen ovale (FO) was patent with very little blood flow through (Fig. 1A). The left atrium (LA) was dilated and non-contractile (Fig. 1B, Supplementary Video S1). Echocardiography showed a highly echogenic mass measuring 10×5 mm in the LA appendage, which was not mobile. The mass was suspected to be a thrombus (Fig. 1C, Supplementary Video S2). Blood tests indicated a high B-type natriuretic peptide (BNP) level (1,102.8 pg/mL) and a mildly elevated D-dimer level (2.27 µg/mL). There was no decrease in platelet count (279×103 /µL) or increase in clotting time (prothrombin time/international normalized ratio: 1.05, activated partial thromboplastin clotting time [APTT]: 29.9 s). The levels of fibrinogen (326 mg/dL), antithrombin-III (65%), protein C (35%), and protein S (68%) were within normal limits for neonates. There was no maternal history of systemic lupus erythematosus or antiphospholipid antibody syndrome, which could have affected neonatal formation of intracardiac thrombi.
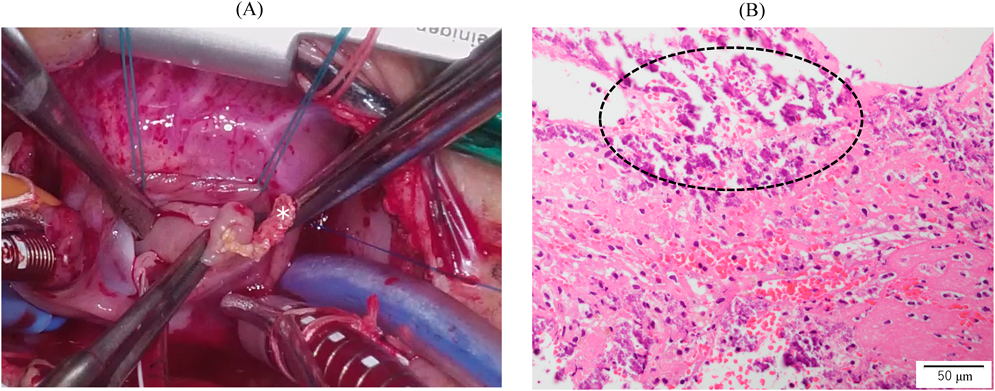
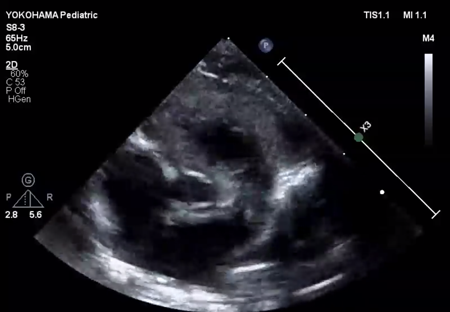
We administered diuretics as well as continuous infusion of olprinone and unfractionated heparin. Continuous infusion of heparin was started at 10 U/kg/hr and gradually increased to 20 U/kg/hr, but the APTT was only slightly prolonged to 36.0 seconds on day 9. Daily echocardiography showed gradual improvement in LV function. Olprinone and diuretics were discontinued by day 9, because the LV ejection fraction improved to 65% and the LA size normalized (Fig. 1D, Supplementary Video S3). Marked mobility of the mass appeared in parallel with the restoration of LA contractility on day 10 (Supplementary Video S4). We performed a surgical thrombectomy on cardiopulmonary bypass so as to avoid systemic embolism. A white structure adhering to the LA appendage was excised (Fig. 2A), and pathological diagnosis was a calcified thrombus (Fig. 2B). The postoperative course was uneventful. Low-dose aspirin was started on the 3rd postoperative day. The patient was followed up on an outpatient basis, and aspirin was discontinued at 4 months postoperatively. At the time of this publication, the patient is 2 years old, and she has experienced no recurrent clots or embolic episode thus far.
Informed consent was obtained from the patient’s guardian for the publication of this case study.
Intracardiac thrombus is rare in neonates, and most reports are related to intravascular placement of a catheter, which causes thrombus formation in the right heart.1, 6) There are few reports of thrombosis within the LA appendage in neonates; the circumstance is relatively common in adults and often associated with atrial fibrillation and cardiomyopathy. Decreased cardiac output and congested blood flow in the LA could occur in any of neonatal cases. Their etiologies vary widely. Al Dhahri et al.1) and Abid et al.2) reported cases of coarctation of the aorta with their LA enlarged. Russo et al.3) reported a left atrial appendage thrombus complicating sustained supraventricular tachycardia. Also, Rohit et al.4) reported a case of septic shock in a preterm and very low-birth-weight infant. Fujiwara et al.5) reported a case with a dilated LV and LA, which was suspected in association with fetal LV dysfunction. In our present case, LV dysfunction with the dilated and stunned LA also suggested the presence of blood stagnation within the LA.
A definite etiology of transient cardiac dysfunction remained unknown; we consider a couple of potential causes. The first is transient myocardial ischemia caused by hypoxia and acidosis due to perinatal distress, which leads to impaired cardiac function and worsened circulatory dynamics after birth. This patient had several risk factors for neonatal myocardial injury, as previously reported, namely postnatal respiratory distress as well as elevated CK and LDH.7) The second possible cause is an intrauterine premature restriction of the FO (PRFO). We were unable to make a definitive diagnosis for this, because there were no records of fetal echocardiography. Postnatal echocardiographic findings suggestive of PRFO include persistent pulmonary hypertension, enlargement and dysfunction of the RV, and poor progress of the LV. In this scenario, findings of persistent pulmonary hypertension beyond 60 hr after birth and minimal inter-atrial shunting were consistent with PRFO findings. Unlike a typical case, her biventricular volumes were balanced. Still, it has been reported that PRFO varies widely in terms of ventricular size and function. Uzun et al.8) surveyed clinical characteristics of restrictive fetal FO in a structurally normal heart, and postnatal LV dysfunction was found in 3 out of 21 cases.
Treatment of an intracardiac thrombus in children includes anticoagulation (low molecular weight heparin, unfractionated heparin, warfarin), fibrinolysis (tissue plasminogen activator), and surgical resection.6, 9) Although surgical resection is a rare occasion, it should be considered appropriate when the thrombus is in the left heart and the risk of systemic embolism is high,6) as was the case in our patient. Size and shape are also important factors when investigating intracardiac thrombi. Thrombi that are large, pedunculated, and mobile may be more prone to embolization.6, 10, 11) In the present patient, the thrombus was not mobile on admission, but mobility became pronounced with improved LV function (Supplementary Video S4). Therefore, we decided to carry out surgical option. Daily echocardiography helped detect the increased risk of embolism and indicated the optimal timing for surgery.
In conclusion, we reported a neonatal patient with a thrombus in the left atrial appendage associated with transient LV dysfunction. Even without congenital heart disease, neonates with impaired left heart function are at risk of developing thrombi in the LA appendage. Serial echocardiography helped assess the indications for surgical thrombectomy.