The Ross procedure is an excellent option for aortic valve replacement in neonates, infants, children, and adolescents. There are significant benefits in the younger cohort due to the growth potential, very good durability, the ready availability of a properly sized valve for the replacement, and freedom from lifelong anticoagulation. As children grow up, the excellent hemodynamics of a Ross procedure, as well as the freedom from anticoagulation allows for full participation in sports. While growth is less of an issue, adolescents with aortic valve disease also benefit from the advantages of a Ross procedure. This patient group also includes women contemplating pregnancy, who benefit particularly from the lack of the need for anticoagulation. The Ross procedure, introduced by Dr. Donald Ross in 1967,1) has been proven to be one of the best options for this population. Since then, the Ross procedure has been performed for over 50 years. The number of Ross procedures has increased dramatically,2) showing excellent long-term survival, long-term autograft durability, and high rates of freedom from reoperation.
However, the Ross procedure proposes the technical challenges, higher mortality and morbidities in younger age groups, and necessity of reintervention for pulmonary allograft, especially in children.3–5) In this article, we review the Ross procedure in children in terms of indications, surgical technique, and early and long-term outcomes, including data form the most recent the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database report, the largest patient cohort in North America, single-center institutional studies, multicenter registry data, and our institutional experiences. For our institutional data, a retrospective single center cohort study was performed, including 240 of consecutive patients aged younger than 18 years undergoing Ross or Ross/Konno procedures between 1991–2013 at the University of Michigan Congenital Hear Center. The data was collected by electric chart review. The survival status was obtained by public record query and follow up data was obtained by cardiology records. To evaluate outcomes after the Ross procedure, the long-term survival, reintervention rates were calculated using Kaplan–Meier curves and Cox proportional hazard models. The outcomes were stratified by age spectrums with infant (<1 year), children (1–12 years), and Adolescent (12–18 years).3)
Indication and Timing for Ross in Pediatric Patients
The STS data reported 2,805 children undergoing the Ross procedure between 2000 and 2018, including the primary diagnosis of valvar aortic stenosis (AS) (n=873, 31%), aortic insufficiency (AI) (n=706, 25%), Aortic Stenosis and Insufficiency (ASI) (n=688, 25%), subvalvar AS (n=231, 8%), and others. For neonates and infants, valvar AS was the most common diagnosis (54% in neonates [<30 days], 47% in infants [30–365 days]). In older patients, the proportion of AI and ASI is higher (54% in children [1–12 years], 65% in adolescents [13–17 years]). Although 87% of neonates underwent the Ross procedure without any previous cardiac intervention, 56% of infants, 48% of children, and 33% of adolescents underwent Ross after more than one previous cardiac procedure, assuming the history of aortic valve intervention are partly contributing to the deviation of the indications (Table 1).
Table 1 Patient characteristics and outcomes| Variables, n (%) | All | Neonate (<30days) | Infant (30–365 days) | Child (1–12 years) | Teenagers (13–17 years) | p value |
|---|
| N=2,805 | N=163 | N=448 | N=1,444 | N=750 |
|---|
| Primary diagnosis | | | | | | |
| Aortic stenosis | 873/2790 (31.3) | 88/162 (54.3) | 208/445 (46.7) | 389/1435 (27.1) | 188/748 (21.5) | <0.01 |
| Aortic insufficiency | 706/2790 (25.3) | 7/162 (4.3) | 44/445 (9.9) | 405/1435 (28.2) | 250/748 (33.4) | <0.01 |
| Aortic stenosis and insufficiency | 688/2790 (24.7) | 24/162 (14.8) | 61/445 (13.7) | 372/1435 (25.9) | 231/748 (30.9) | <0.01 |
| Status | | | | | | <0.01 |
| Elective | 955/1101 (86.7) | 24/65 (36.9) | 115/178 (64.6) | 523/554 (94.4) | 293/304 (96.4) | |
| Urgent | 132/1101 (12.0) | 36/65 (56.9) | 59/178 (33.2) | 25/554 (4.5) | 11/304 (3.6) | |
| Emergent/Salvage | 14/1101 (1.3) | 4/65 (6.2) | 4/178 (2.2) | 6/554 (1.1) | 0/304 (0) | |
| Previous cardiac procedures | | | | | | <0.01 |
| No | 1536/2718 (56.5) | 136/157 (86.6) | 190/437 (43.5) | 720/1398 (51.5) | 490/726 (67.5) | |
| Yes | 1182/2718 (43.5) | 21/157 (13.4) | 247/437 (56.5) | 678/1398 (48.5) | 236/726 (32.5) | |
| Concomitant procedures | | | | | | |
| Aortic coarctation | 29 (1.0) | 18 (11.0) | 6 (1.3) | 5 (0.3) | 0 (0) | <0.01 |
| Aortic arch | 163 (5.8) | 58 (35.6) | 55 (12.3) | 40 (2.8) | 10 (1.3) | <0.01 |
| Mitral | 179 (6.4) | 23 (14.1) | 69 (15.4) | 70 (4.9) | 17 (2.3) | <0.01 |
| Konno | 1013 (36.1) | 139 (85.3) | 326 (72.8) | 460 (31.9) | 88 (11.7) | <0.01 |
| Outcomes | | | | | | |
| Operative mortality | 116/2790 (4.2) | 39/162 (24.1) | 50/446 (11.2) | 21/1433 (1.5) | 6/749 (0.8) | <0.01 |
| Mechanical circulatory support | 120 (4.3) | 37 (22.7) | 46 (10.3) | 28 (1.9) | 9 (1.2) | <0.01 |
| Unplanned reoperation | 149 (5.3) | 24 (14.7) | 44 (9.8) | 59 (4.1) | 22 (2.9) | <0.01 |
| Respiratory insufficiency requiring mechanical ventilation or reintubation | 187 (6.7) | 62 (38.0) | 75 (16.7) | 40 (2.8) | 10 (1.3) | <0.01 |
| Bleedind requiring reoperation | 96 (3.4) | 11 (6.7) | 26 (5.8) | 38 (2.6) | 21 (2.8) | <0.01 |
| Reprinted with permission from Reference 2). |
Congenital AS is an ideal indication for the Ross procedure. It is one of the five most common congenital heart diseases, and it is classified with valvar (70%), supravalvar (5–10%), and subvalvar stenosis (10–20%). Approximately 10% of congenital AS present as critical AS in their newborn or infant period. In utero, systemic cardiac output is maintained through patent ductal arteriosus (PDA), and severe AS is well tolerated. After birth as the PDA closes, patients may present with acute congestive heart failure with depressed left ventricular (LV) function due to the significant afterload and decreased cardiac output. Thus, in the postnatal period, the patency of PDA with prostaglandin infusion is critical and urgent surgical intervention may be required. Per the STS report, the Ross procedure was performed more in urgent situations in neonates (57%) and infants (33%), compared to children (4.5%) or teenagers (3.6%) (Table 1). In non-critical AS, as the stenosis progresses, symptoms such as angina, syncope, or heart failure may appear. Any of these symptoms with hemodynamically significant pressure gradient across the aortic valve (peak-to-peak pressure gradient of greater than 50 mmHg) is an indication for aortic valve intervention. For asymptomatic patients, severe stenosis with a peak-to-peak gradient across the aortic valve of 75 mmHg is an indication for intervention. Therapeutic intervention should be considered if there is any evidence of progressive LV hypertrophy, ischemia, dysfunction, or arrhythmia. Mild to moderate AS (peak-to-peak pressure gradient of less than 40 mmHg) with an asymptomatic patient should be managed with regular follow-up with an echocardiogram.
A Strategy of Aortic Valve Intervention for Congenital AS
A choice of initial interventional approach for congenital AS is controversial. A catheter-based balloon valvotomy and open surgical valvuloplasty are often palliative and reinterventions may be required, although both methods can provide equivalent survival outcomes.6) Data showed survival after aortic valve intervention at ten years was approximately 90%, freedom from operation at ten years for balloon valvuloplasty was 27–36%, compared to 65–66% for surgery, and freedom from aortic valve replacement at ten years was 60% for balloon valvuloplasty compared to 79% for surgery.6, 7) These data suggest that surgical intervention can minimize the need for reoperation and effectively delay aortic valve replacement, including the Ross procedure, compared to balloon valvuloplasty. Since the higher mortality and necessity of reintervention for pulmonary valve after the Ross procedure in infants is well recognized,2, 3, 8) delaying the Ross procedure would benefit this population.
Surgical Techniques for Ross Operation
General Technique
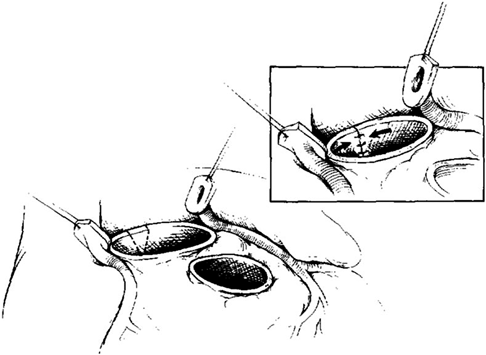
A standard cardiopulmonary bypass with ascending aortic and bicaval cannulation is performed. Cold blood cardioplegia and topical cooling are used for myocardial protection. When patients need arch reconstruction, deep hypothermic circulatory arrest or regional cerebral perfusion can be utilized. After cardioplegic arrest, the aortic root was open 2 to 3 mm above the sinotubular junction, and the aortic valve should be assessed for possible aortic valve repair before resecting the valve leaflets and aortic wall or harvesting the pulmonary autograft. The pulmonary artery is transected at its bifurcation, and the pulmonary valve annulus size is approximated with calibrated dilators before the excision of the autograft. If the valve is not amenable to repair, the diseased aortic valve is excised, and the coronary ostia were removed with buttons of aortic tissue. The pulmonary autograft is then harvested with a 2- to 3-mm muscle cuff, and care was taken to avoid injury to the underlying left main coronary artery and the septal perforators posteriorly. Aortic anulus tailoring is performed if there was a more than 2 mm larger aortic annulus than pulmonary autograft.9) It was begun by excising a triangular wedge of tissue from the aortic valve annulus at the level of the commissure between the left and noncoronary cusps, extending into the anterior leaflet of the mitral valve (Fig. 1). The V-shaped defect was then reapproximated over a calibrated dilator, which was adjusted to achieve a final diameter of 2 mm less than that of the pulmonary annulus. The edges are reapproximated with interrupted, pledgeted, horizontal mattress polypropylene sutures. The autograft is then sutured to the tailored aortic root with a continuous polypropylene suture beginning below the origin of the left coronary artery. The coronary arteries are then implanted into the facing sinuses of the autograft. The distal autograft anastomosis is performed, and the cross clamp removed. The right ventricular outflow tract is reconstructed with an appropriately sized cryopreserved pulmonary allograft while the patient was being rewarmed.
Ross/Modified Konno Procedure
An aortic annulus enlargement procedure is commonly needed in neonates and in some infants. The modified Konno technique is used for aortic annulus enlargement entailing an incision through the aortic annulus and into the septum between the right and left coronary cusps. This septal incision is partial thickness, and the endocardium of the right ventricular outflow tract is maintained intact. If subaortic stenosis is present, a wedge resection of the septal can also be performed. The autograft was anastomosed proximally to the enlarged annulus. In the region of the divided aortic annulus and septal incision, the annulus of the autograft was sewn to the endocardium of the right ventricular outflow tract. In our institutional experience, the Ross/modified Konno procedure was performed in 78 (33%) patients (n=240) between 1991 and 2013. Significantly, 68% (30 out of 44) of infants required the Ross/modified Konno procedure. The mean size differential between the aortic and pulmonary annuli was 5.7±1.0 mm (4.2 to 7.4 mm).10) Per the STS report, between 2000 and 2018, the Ross/Konno procedure was performed on 1013 patients (36.1%), including 139 neonates (85.3%) and 326 infants (72.8%) (Table 1).
Ross Modification for Late Autograft Dilation
Although initially, Dr. Ross performed the Ross procedure with sub-coronary autograft implantation, more recently full root replacement is more popular since the operative technique is more easily reproducible and the valve more consistently competent due to the preserved cylindrical geometry of aortic root.11) The data show a significant advantage of full root replacement over the subcoronary technique in avoiding regurgitation.12) However, late autograft dilation with an unsupported autograft has been well recognized.13) Annular and sinotubular junction dilation was found to distort the coaptation of the leaflets and cause neoaortic valve regurgitation. Various surgical modifications have been reported to avoid late autograft dilation and regurgitation.14–18)
In children, these technical modifications may not be applicable because the external support may preclude the potential of somatic growth, which is one of the advantages of the Ross procedure in children. Although there is no specific age or body weight cutoff to utilize an external support technique, if the autograft is 19 mm or greater, we use a polyethylene terephthalate (Dacron) Valsalva graft 3–4 mm larger in diameter than the autograft to wrap the autograft to help prevent late dilatation. Multi-institutional data showed excellent mid-term outcomes in patients between 10–35 years old with a supported Ross. These data showed that 98% of patients had mild or less regurgitation with a median sinus size Z-score of 1.4 with a median follow-up of 3.5 years.19) Another option to support the autograft in younger patients is to utilize the original inclusion technique. Ozturk et al. reported that they performed the Ross procedure with the inclusion technique as young as three years old.17)
Excellent short outcomes have been reported in the Ross procedure in the young adult population with 0.4–2.7% of operative mortality.20) In children, it is well recognized that there is a higher risk of operative mortality.3, 4, 8) Although the STS data showed the improving early operative mortality in neonates and infants over the two decades (17.2% from 2000 to 2012 and 11.6% from 2013 through 2018), the early mortality in neonate and infant remains high compared to children (2.1%) and adolescents (1.2%). The risk factors of early mortality were neonatal age (OR 3.0 [1.9–4.8, p<0.01]), concomitant mitral, aortic, or Konno procedure (OR 3.1 [1.1–8.7], p=0.03), low volume center (OR 2.1 [1.1–3.9], p=0.03).3, 8)
The postoperative mechanical circulatory support incidence rate was higher in neonates and infants than in children or adolescents. The overall incidence of mechanical circulatory support after the Ross procedure was 4.3%, with 22.7% in neonates and 10.3% in infants.2) A meta-analysis showed that the rate of mechanical circulatory support after the Ross procedure in neonates was 15% (95% CI 5%–28%).8) The operative mortality in the mechanical circulatory support group was 29%, consistent with another study.4) The other complications, including unplanned reoperation, respiratory failure, postoperative bleeding, or arrhythmia, are also higher in the younger age group (Table 1).2, 3)
Survival Outcomes
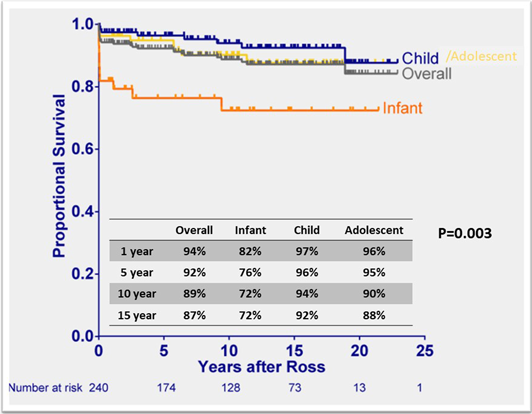
Excellent long-term survival outcomes after the Ross procedure have been published, with 87–95% survival at 15 years (Table 2).3–5, 13, 21, 22) The survival rates stratified by age groups showed consistently lower survival in the neonate/infant group compared to children or adolescents, showing 71–80% survival rates after ten years in neonate/infant (Fig. 2).3, 4) Although early mortality has improved over the last two decades,2) the nature of aortic valve disease including patients presenting in extremis as the PDA closes, comorbidities, a highly technical operation, and challenging perioperative management all contribute to lower survival. Critical congenital AS often also with complex left heart disease, such as endocardial fibroelastosis, abnormal mitral valve structures, left ventricular outflow obstruction, or impaired systolic and diastolic left ventricular function.2) Conversely, children and adolescents tend to be stable clinically, presenting as outpatient cases at the time of surgery with a low proportion of the concurrent procedures.
Table 2 Reviews of long-term outcomes after the Ross procedure by the other literature| Authors | Number of patients | Patient age | 10-year survival (%) | 15-year survival (%) | 10 years Freedom fron reintervention for LVTO (%) | 10 years Freedom from reintervention for RVOT (%) |
|---|
| El-Hamamsy I, et al 13) | 108 | 18–69 years old | 97 | 95 | 97 | 83 |
| Sievers HH, et al 21) | 630 | mean 45 years old | 95 | 87 | 96 | 97 |
| David TE, et al 22) | 212 | 28–41 years old | 98 | 95 | 95 | 98 |
| Fricke TA, et al 25) | 443 | 15–66 years old | 99 | 95 | | 98 |
| Nelson JS, et al 3) | 240 | <18 years old | 89 | 87 | 75 | 77 |
| Bansal N, et al 4) | 305 | 0–70 years old | 92 at 8 years | N/A | 79 at 8 years | 85 at 8 years |
| Donald JS, et al 5) | 140 | children | 92.5 | N/A | 86 | 74 |
Reintervention
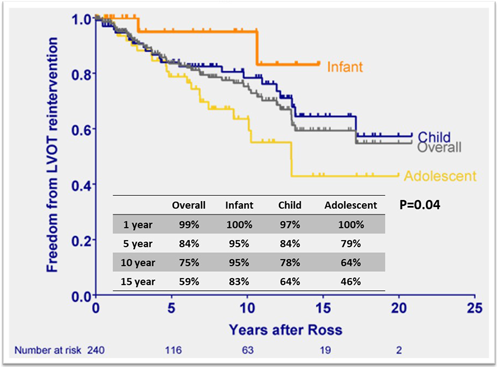
Reinterventions after the Ross procedure are a key metric in assessing the role of the procedure since it converts a single valve problem into two valve disease; the aortic and pulmonary valves both become at risk of reintervention. The main reasons for reintervention are autograft valve regurgitation, autograft root dilatation with or without AI, and pulmonary allograft dysfunction. Freedom from left ventricular outflow tract (LVOT) reintervention at ten years has been reported to range from 75% to 97%, indicating a cumulative risk of 1–1.5%/ patient-year20) (Table 2). Using age-stratified data, freedom from reintervention for LVOT was highest in the infant population with 83–100% at 5–15 years (Fig. 3).3, 4) On the contrary, the adolescent group tended to have higher LVOT reintervention rates compared to other groups, which trend was observed as age increased. While there was no significant difference in age based on the adjusted hazard ratio, over 40% underwent LVOT intervention within ten years postoperatively in an adolescent group.4) The most common indications for LVOT reintervention were neo-aortic root dilation, aortic insufficiency, or both.3, 4) Between 2001 and 2021, 40 patients were presented for LVOT reintervention after our institution’s Ross procedure in childhood. Among them, 25 patients underwent valve-sparing root replacement with root remodeling (n=7), reimplantation (n=11), and remodeling plus annuloplasty ring (n=7) with 96% operative survival.23) One would be hopeful that the recent addition of autograft wrapping in the older child and adolescent Ross procedures will result in fewer autograft reintervention in this age group.
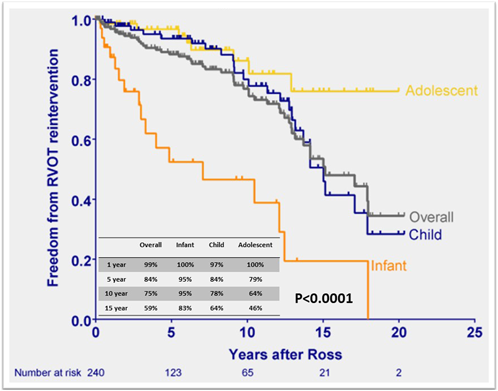
The rate of pulmonary allograft dysfunction after the Ross procedure varies among reports, depending on how dysfunction is defined. Recent data showed favorable durability of pulmonary valve function after the Ross procedure with over 95% freedom from reintervention of right ventricular outflow tract (RVOT) at 20 years in adult populations.24, 25) In children, the overall freedom from RVOT reintervention has been reported to be 77–85% at ten years (Table 2).3–5) Unlike LVOT, not surprisingly the infant population demonstrated shorter durability of the pulmonary allograft, showing 52–58% freedom from RVOT reintervention (Fig. 4).3, 4) The younger age and smaller pulmonary allograft (<14 mm) are shown as the significant risk factors for pulmonary allograft reintervention.3, 26) Although with the advent of catheter-based pulmonary valve implantation, pulmonary valve dysfunction is being mitigated, novel surgical strategy will be warranted to avoid RVOT reintervention, especially in the infant population.
These contemporary data show the increased utilization of the Ross procedure and improved survival outcomes over the decades. Outcomes after the Ross procedure in children vary by age at the operation. The Ross procedure in the neonate/infant population remains challenging, with higher early mortality due to the complex left heart disease and the severity of the disease. However, this is balanced by the better long-term durability of the neoaortic valve in infants, but in the context of a higher reintervention rate on the RVOT in this younger age population. Further study, perioperative management strategy, or surgical innovation will lead to improve the outcomes of the Ross procedure in the youngest children. For older children and adults, the Ross procedure performs well, albeit with a higher rate of autograft reintervention than neonates and infants, which will hopefully be mitigated by wrapping techniques when the diameter of the autograft allows.
Overall, the Ross procedure is excellent option for neonates, infants, children and adolescents encompassing many of the characteristics of an ideal aortic valve replacement, including excellent long-term survival, relatively low rates of reintervention, low thrombogenicity and the lack of the need for anticoagulation, ready availability of the proper sized valve, growth potential, and excellent hemodynamic performance.
謝辞Acknowledgments
We thank the Japanese Society of Pediatric Cardiology and Cardiac Surgery for allowing us to publish the review article “The Ross procedure in pediatric patients” by The Journal of Pediatric Cardiology and Cardiac Surgery, led by Drs. Ohuchi, Yamagishi, and Suzuki.
Conflicts of Interest
All authors have no conflict of interest.
Author Contributions
JM: Conceived and designed the study, collected data, and wrote paper
SHT: Wrote and review paper
RGO: Conceived and designed the study, wrote and review paper
引用文献References
1) Ross DN: Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967; 2: 956–958
2) Rowe G, Gill G, Zubair MM, et al: Ross procedure in children: The Society of Thoracic Surgeons Congenital Heart Surgery Database Analysis. Ann Thorac Surg 2023; 115: 119–125
3) Nelson JS, Pasquali SK, Pratt CN, et al: Long-term survival and reintervention after the Ross procedure across the pediatric age spectrum. Ann Thorac Surg 2015; 99: 2086–2094, discussion, 2094–2095
4) Bansal N, Kumar SR, Baker CJ, et al: Age-related outcomes of the ross procedure over 20 years. Ann Thorac Surg 2015; 99: 2077–2083
5) Donald JS, Wallace FRO, Naimo PS, et al: Ross operation in children: 23-year experience from a single institution. Ann Thorac Surg 2020; 109: 1251–1259
6) Vergnat M, Asfour B, Arenz C, et al: Aortic stenosis of the neonate: A single-center experience. J Thorac Cardiovasc Surg 2019; 157: 318–326.e1
7) Siddiqui J, Brizard CP, Galati JC, et al: Surgical valvotomy and repair for neonatal and infant congenital aortic stenosis achieves better results than interventional catheterization. J Am Coll Cardiol 2013; 62: 2134–2140
8) Rajab TK, Zorrilla-Vaca A, Kavarana MN, et al: Ross operation in neonates: A meta-analysis. Ann Thorac Surg 2022; 113: 192–198
9) Durham LA 3rd, desJardins SE, Mosca RS, et al: Ross procedure with aortic root tailoring for aortic valve replacement in the pediatric population. Ann Thorac Surg 1997; 64: 482–486
10) Ohye RG, Gomez CA, Ohye BJ, et al: The Ross/Konno procedure in neonates and infants: Intermediate-term survival and autograft function. Ann Thorac Surg 2001; 72: 823–830
11) Knott-Craig CJ, Elkins RC, Stelzer PL, et al: Homograft replacement of the aortic valve and root as a functional unit. Ann Thorac Surg 1994; 57: 1501–1505
12) Elkins RC: The Ross operation: A 12-year experience. Ann Thorac Surg 1999; 68 Suppl: S14–S18
13) El-Hamamsy I, Eryigit Z, Stevens LM, et al: Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: A randomised controlled trial. Lancet 2010; 376: 524–531
14) Mazine A, Ghoneim A, El-Hamamsy I: The Ross procedure: How I teach it. Ann Thorac Surg 2018; 105: 1294–1298
15) Juthier F, Banfi C, Vincentelli A, et al: Modified Ross operation with reinforcement of the pulmonary autograft: Six-year results. J Thorac Cardiovasc Surg 2010; 139: 1420–1423
16) Varrica A, Satriano A, Frigiola A, et al: Autograft wrapping reinforcement in adolescents undergoing ross operation: A tailored coat. Ann Thorac Surg 2022; 114: 866–871
17) Ozturk M, Tongut A, Hanabergh SS, et al: The Ross procedure with the inclusion technique. Oper Tech Thorac Cardiovasc Surg 2022; 27: 414–422
18) Matsushima S, Abeln KB, Karliova I, et al: Suture annuloplasty and simplified root wrapping in the full root Ross operation. Ann Thorac Surg 2019; 107: e361–e363
19) Riggs KW, Colohan DB, Beacher DR, et al: Mid-term outcomes of the supported Ross procedure in children, teenagers, and young adults. Semin Thorac Cardiovasc Surg 2020; 32: 498–504
20) Chauvette V, Lefebvre L, Chamberland M-È, et al: Contemporary review of the Ross procedure. Struct Heart 2021; 5: 11–23
21) Sievers HH, Stierle U, Petersen M, et al: Valve performance classification in 630 subcoronary Ross patients over 22 years. J Thorac Cardiovasc Surg 2018; 156: 79–86.e2
22) David TE, Ouzounian M, David CM, et al: Late results of the Ross procedure. J Thorac Cardiovasc Surg 2019; 157: 201–208
23) Hobbs RD, Schultz ML, Loney ML, et al: Valve-sparing root replacement after the Ross procedure. J Thorac Cardiovasc Surg 2023; 165: 251–259
24) Chauvette V, Bouhout I, Tarabzoni M, et al: Canadian Ross Registry: Pulmonary homograft dysfunction after the Ross procedure using decellularized homografts-a multicenter study. J Thorac Cardiovasc Surg 2022; 163: 1296–1305.e3
25) Fricke TA, Skillington PD, Shi WY, et al: Pulmonary valve function late after Ross procedure in 443 adult patients. Ann Thorac Surg 2020; 109: 1127–1131
26) Friedman KG, Salvin JW, Wypij D, et al: Risk factors for failed staged palliation after bidirectional Glenn in infants who have undergone stage one palliation. Eur J Cardiothorac Surg 2011; 40: 1000–1006