This study involved 12 infants who were under observation for ≥6 months after surgery from April 2014. All the patients had undergone medical treatment for ≥4 weeks with limited effect and therefore required surgical treatment. The patients were aged 30 days to 2 years (mean age, 9 months; median age, 8 months) and included 6 males and 6 females, 5 of whom had underlying diseases other than cardiovascular diseases (3 with trisomy 21, 1 with refractory hydrocephaly, and 1 with Noonan syndrome). The patients had severe congenital cardiac diseases such as common ventricle, tetralogy of Fallot (TOF) with pulmonary atresia, and Ebstein anomaly (Table 1). Lymph flow testing and treatment included lymphography (LG; indocyanine green [ICG], Lipiodol; detailed below) and lymphaticovenular anastomosis (LVA). In the patients without a right-to-left shunt, LG was performed, and LVA was indicated in patients with a right-to-left shunt, suspected congenital diseases, and edema (Fig. 1). All the surgeries were performed under general anesthesia (total intravenous anesthesia [TIVA]). ICG fluorescence imaging, LG, and LVA were performed with the approval of the institutional ethics committees of our hospital.
Table 1 Patient list| No | Age (month-old) | Sex | Weight at lymph surgery (g) | Diagnosis | Cardiac anomaly | Other comorbidities | Previous cardiac/vascular surgery | Treatment | Post-op course | Outcome |
|---|
| 1 | 1 | Male | 2.8 | CT | Single ventricle | | PA banding | LVA | PR | Death due to respiratory distress |
| | | | | Vertical vein stenosis | | Vein stent | | | 3 months post-surgery |
| | | | | AVSD | | | | | |
| 2 | 1 | Female | 0.86 | CT | | ELBWI (518 g), hydrocephalus | | LG | CR | Hospitalization due to hydrocephalus |
| 3 | 1 | Male | 2.9 | CTA | TAPVR | | PA banding | LG | PR | |
| | | | | AVSD | | | | | |
| 4 | 2 | Female | 2.5 | CT | | | | LVA | CR | |
| 5 | 4 | Female | 3.3 | CT | | | | LVA | CR | |
| 6 | 6 | Female | 4.6 | CTA | TOF | 21 trisomy | | LVA | PR | Death due to sepsis |
| | | | | | | | | | 1 year post-surgery |
| 7 | 11 | Female | 3.5 | CT, PLE | | Noonan synd. | | LVA | PR | |
| 8 | 12 | Male | 3.3 | CTA | Ebstein, TAPVR | 21 trisomy | Starnes | LVA | PR | Death due to respiratory distress |
| | | | | | | | | | 1 year post-surgery |
| 9 | 13 | Male | 3.8 | CA, PLE | TAPVRASD | | ASD direct closure | LVA | NR | Death due to respiratory distress |
| | | | | | | | | | 2 months post-surgery |
| 10 | 14 | Female | 5.2 | CT | TOF | 21 trisomy | B-T shunt | LVA | NR | Death due to sepsis |
| | | | | | | | | | 8 months post-surgery |
| 11 | 15 | Male | 7.3 | CTA | | Lymphang iomatosis | | LG | PR | |
| 12 | 30 | Male | 10 | CT | Aortic stenosis | | Aortic plasty | LG | CR | |
| Case result in death were more likely on patients with refractory chylothorax due to congenital severe heart diseases. We perform surgical treatment only in cases with medical treatments at least 1 month did not cure the chyle leakage. CT: chylothorax, CTA: chylothorax and abdomen, CA: chyloabdomen, PLE: protein loosing enteropathy. AVSD: atrioventricular septal defect, TAPVR: total anomalous pulmonary venous return, TOF: tetralogy of Fallot, PA: pulmonary artery, ELBWI: extremely low body weight infant. LVA: Lymphatic venous anastomosis, LG: Lymhangiography with Lipiodol. PR: partial response, CR: complete response, NR: no response. |
ICG Fluorescence Imaging
ICG injection fluid (Diagnogreen 0.5%; Daiichi Pharmaceutical, Tokyo, Japan) was diluted to 10 mL of solution or 5% fructose solution, and 0.05 mL was subcutaneously injected to the dorsal pedis and dorsal manus. Following the injection, an infrared camera was used to thoroughly observe the body surface. The skin directly above the linear pattern of lymph flow or circular accumulations in the inguinal region indicating the lymph nodes was marked with a permanent marker.
Lipiodol Lymphography (LG)
The inguinal lymph nodes on one or both sides were punctured with a 27-G needle under ultrasonography or directly viewed through a skin incision. Lipiodol 480 10 mL (Fuji Pharma Co. Ltd., Tokyo, Japan) was manually injected, very slowly, and the forefront was identified on radiographic fluoroscopy; this should be done under magnification in small infants. The injection was stopped as soon as the thoracic duct was visualized (approximately 0.2 mL). As previously mentioned, patients with a right-to-left shunt are at a risk of brain infarction; thus, patients with a shunt detected on echocardiography are not suitable for this method (Fig. 1).
Lymphaticovenular Anastomosis (LVA)
Lymphatic pathways imaged under ICG near the great saphenous vein were selected as the operative area; 0.1 mL of 0.5% concentrated lidocaine fluid with Bosmin diluted 20,000 times was subcutaneously injected as a local anesthetic, and a 1- to 2-cm incision was made to the skin. The subcutaneous tissues were carefully dissected under microscopic magnification to expose the collecting lymph vessel and vein. Approximately 2–6 11-0 or 12-0 nylon sutures were applied for anastomoses. When complete patency of the anastomosis was verified, the skin was sutured using 5-0 absorbent monofilaments.
The 3 possible pathologies for the onset of chylothorax and chylous ascites are presented herein. A complex combination of these pathologies is involved in this disease, making it refractory in many cases. Treatment strategies depend on the facility, attending physician, and patient’s conditions. Medical therapy may be successful in these cases; thus, surgical therapy is performed only after conservative efforts have failed.2) Fasting (or medium-chain triglyceride milk and low-lipid diet) from an early stage, and octreotide, steroid, and fibrogammin are gradually administered,3, 4) and surgical treatment is considered after 1 month if conservative therapy does not stop leaks. However, we continue to conduct lymph flow testing to gain a better understanding of the pathology. Clinical findings may exhibit symptoms of superficial edema, protein-losing enteropathy, and pericardial effusion collection.5, 6)
Traumatic Lymph Duct (Thoracic Duct) Injury
This may occur iatrogenically or traumatically, and may occur after thoracic surgery; diagnosis is made relatively early.7) It may stop with conservative therapy or may persist for an extended period.8) This injury is a good indication for LG.
Congenital Chylothorax and Chylous Ascites
These conditions are possibly related to abnormally formed pathways or underdeveloped lymph ducts and may be complicated by lymph duct malformations (lymphangiomas) in the abdominal or thoracic cavity. They may be observed during the fetal stage with fetal hydrops or may develop shortly after birth.9)
Thoracoabdominal Fluid Accompanying Phlebostasis
This may occur in cases of postoperative venous hypertension following Fontan or Glenn surgery or in total anomalous pulmonary venous return (TAPVR) causing pulmonary vein stenosis.10) Venous pressure is practically impossible to decrease when considering the circulatory dynamics, and thoracoabdominal fluid drainage may be difficult. This condition may be caused by calcification or venous thrombosis because of long-term central venous catheter placements.
Testing Methods
Magnetic resonance imaging (MRI) and computed tomography (CT) are applied for morphological evaluation, and LG (scintigraphy, ICG fluorescence imaging, and LG using contrast agents) is used to evaluate lymphatic flow.
Lymphatic scintigraphy has a low resolution and is becoming less frequently used in recent years. Single-photon emission computed tomographic CT is used increasingly for deep lymph evaluation11) but not frequently because it exposes the patient to radioactive substances and is less sensitive than MRI.
ICG fluorescence imaging is easy to maneuver, allows immediate observation, does not expose the patient to radiation, and can detect lymph pathways and superficial lymph reflux. Additional observations can be made 1 week after the injection without the need for another subcutaneous injection, which is particularly advantageous for evaluating children and newborns (Fig. 2). Although it is possible to gain detailed information from the drained fluid, other testing methods are often used for treating chest/abdominal fluid.
During LG, a contrast agent is injected directly into the lymphatic system; this is also known as direct LG. In adults, a direct puncture is conventionally made in the lymph ducts on the top of the foot.12, 13) This method is difficult to conduct in children, but direct puncture of the inguinal lymph nodes makes it possible to evaluate central lymphatic flow as well (Fig. 3). However, the procedure requires experience and is performed under general anesthesia with positive pressure ventilation in children; therefore, visualization of the main lymph ducts in the thoracic cavity may sometimes be poor.14) Recently, evaluation with dynamic magnetic resonance LG has allowed a more detailed understanding of the pathology.15–17) Leakage sites and patterns have been reported in patients diagnosed as having congenital chylothorax, demonstrating its high diagnostic value.18)
Treatment Methods
Lymph from the lower limbs flows through the abdominal chyle cistern, past the thoracic duct, and into the venous angle. Central lymph flow disorders cause lymphorrhea, which causes many symptoms, including pleural effusion, ascites, pericardial fluid, protein-losing enteropathy, chyluria, and edema. Although surgery is possible for these conditions, they often require combination therapy.
Accumulated chest/abdominal fluid can be treated by thoracic/abdominal cavity drainage or thoracoabdominal shunt construction.19) Pleurodesis or thoracic duct ligation are surgical approaches for managing leak sites. LG (20) and LVA are typical treatment methods in lymph surgery (Fig. 4).
In LG, a highly viscous contrast agent flows into the thoracic cavity through the lymph vessels. Either direct embolism by the agent itself or selective adhesion evoked inflammatory response in the surrounding tissues at the leakage site is considered the mechanism of LG. Lymphedema is a possible complication that requires attention. We frequently use a method of LG to perform ICG prior to LG (ICG-LG).20) This enables an early prediction of leg edemas after LG without additional injection of contrast agents. Furthermore, Lipiodol can leak into the neurovascular flow in patients with right-to-left shunt and can cause a brain infarction21); hence, we prohibit the use of LG for patients with right-to-left shunts in our hospital. Pulmonary embolism can occur; therefore, we avoid additional injections of Lipiodol after venous inflow is observed. In this study, none of the patients had postoperative respiratory symptoms.
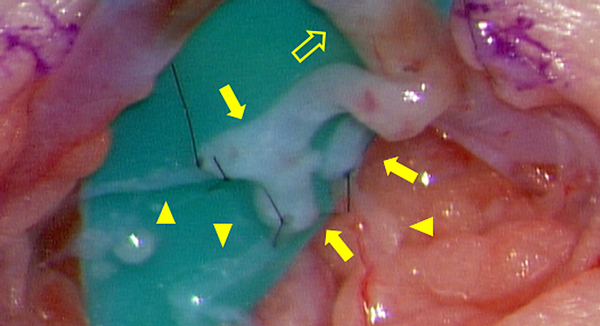
We prefer using LVA to establish lymph flow. This method was developed in Japan primarily for treating adult secondary lymphomas.22) It is a minimally invasive procedure, as the surgical fields are limited only on the superficial layers with small skin incisions; it is possible to perform this procedure under local anesthesia in adults.23) LVA is a relatively natural reconstruction method because the lymph flows into the veins eventually24) (Fig. 5). The underlying mechanism comprises releasing lymph from the lymph tract to the venous system; collecting lymph from the lower limbs into the central lymphatic system (e.g., pelvic lymph tract, chyle cistern, thoracic duct) into the veins, and/or collecting lymph fluid that has refluxed to the body surface from the central system, and/or small quantities of blood with hemostasis factors from the venous system that mix with the lymphatic tract to adhere to the leak site.25)
The success rates in our study were 33% complete remission, 50% partial response, 17% no response, 50% remission, 8% continued hospitalized treatment, and 42% death, with death occurring most often during the observation period. All the patients who died had undergone LVA; however, we observed that the patients who underwent LG had relatively mild disease simply caused by the iatrogenic trauma or who had no right-to-left shunt. Patients who died later had higher rates of TAPVR, TOF, and venous hypertension. Conditions such as not simple traumatic lymph injuries nor congenital chyle-thorax/abdomen, but a combined condition with high venous pressure, might complicate their disease. Furthermore, malnutrition led by lasting chest/abdominal fluid for >6 months could be a poor prognostic factor. In this study, we did not apply additional treatment; however, it may be better to perform additional surgeries in patients with PR with fair general conditions for early improvement. Secondary LG is possible for primary effective LG patients, and secondary LVA is possible for patients with ineffective primary LG or primary LVA.
On the basis of our previous clinical research, we believe that obstruction (stenosis) and the accompanying reflux are important risk factors of lymphedema and other lymphatic diseases.26) For example, lymphatic malformations (i.e., lymphangiomas) have been treated with sclerotherapy or surgical resection; however, little consideration is given to lymph flow in these treatments.27) By contrast, even for fractable micro-cystic lymphatic malformations (cavernous lymphangioma composed of aggregates of small cysts), lymph flow could be reestablished with good results on the basis of flow assessment with ICG fluorescent.28, 29) Treatments based on lymph flow evaluation can also be effective for chest/abdominal fluid. In particular, lymph fluid reflux from the point of obstruction to the subcutaneous areas of the thigh is considered a good indicator for this treatment.
Thoracic duct embolization, a method for treating chest/abdominal fluid, has gained attention recently.14, 17, 30, 31) In this method, a direct puncture is made after visualizing the thoracic duct through contrast imaging. A catheter is placed, after which an embolizing substance (NBCA or coil) is used to embolize the leaking area or main thoracic duct internally. An American team reported relatively good results, but the success rate was lower in children and patients with heart diseases.32) Furthermore, lymph fluid that is blocked from entering the emoblized main thoracic duct may congest in the intestinal tract or thigh, requiring caution to prevent refractory protein-losing enteropathy or lower-extremity lymphedema.
In most of the patients with terminated chyle leak, growth and development improved to normal levels within several months after discharge from the hospital. By contrast, those with complications of congenital lymphangiectasia may have exacerbated symptoms later even if the pleural effusion or edema is completely cured.5) Therefore, in our hospital, even patients who enter remission with medical treatment alone routiney undergo long-term follow-up observation in the lymphology outpatient clinic and are tested and treated as needed.
Lymphatic diseases are undergoing a phase of major change. New techniques allow direct manipulation of the lymph vessels, which has paved the way for the development of more effective and detailed diagnostic and therapeutic methods. Similar to other vascular channel diseases, lymph vessels also have a flow. Lymph fluid is clear and characterized by a “quiet” flow, as its flow is slower than those of other vascular channels, which may be why it has not been sufficiently reported. In addition, future surgical treatments should focus on improved lymph flow in lymphatic diseases. A flow-oriented surgical strategy (or flow-oriented super-micro surgery [FOSS]) is a minimally invasive treatment strategy for evaluating and reestablishing lymph flow.25)
We treat this disease using a team approach in which physicians practicing cardiovascular internal medicine and surgery, intensive care, radiology, surgery, pediatrics and general pediatrics, and plastic surgery apply their knowledge and skill. We have experienced only a few cases, but more data and future case findings must be accumulated to develop precise therapies. We sincerely hope that these data can help young patients with lymphatic diseases and that this manuscript contributes to their future care.
引用文献References
1) Hwang JH, Kim JH, Hwang JJ, et al: Pneumonectomy case in a newborn with congenital pulmonary lymphangiectasia. J Korean Med Sci 2014; 29: 609–613
2) Nadolski G: Nontraumatic chylothorax: Diagnostic algorithm and treatment options. Tech Vasc Interv Radiol 2016; 19: 286–290
3) Schild HH, Strassburg CP, Welz A, et al: Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013; 110: 819–826
4) Lopez-Gutierrez JC, Tovar JA: Chylothorax and chylous ascites: Management and pitfalls. Semin Pediatr Surg 2014; 23: 298–302
5) Hirano H, Mori Y, Takeuchi K: A case of chylothorax and chylous ascites after ten years with the chylopericardium. Medical J Iwate Prefectural Hospital 2000; 40: 81–84 (in Japanese)
6) Rosa GM, Campisi C, Bioccardo F, et al: Chylopericardium: a case report demonstrating utility of lymphography combined with 3D computed tomography for corrective surgical treatment using VATS. Lymphology 2014; 47: 40–43
7) Pillay TG, Singh B: A review of traumatic chylothorax. Injury 2016; 47: 545–550
8) Choh CT, Rychlik IJ, McManus K, et al: Is early surgical management of chylothorax following oesophagectomy beneficial? Interact Cardiovasc Thorac Surg 2014; 19: 117–119
9) Scalzetti EM, Heitzman ER, Groskin SA, et al: Developmental lymphatic disorders of the thorax. Radiographics 1991; 11: 1069–1085
10) Dori Y, Keller MS, Rychik J, et al: Successful treatment of plastic bronchitis by selective lymphatic embolization in a Fontan patient. Pediatrics 2014; 134: e590–e595
11) Yang J, Codreanu I, Zhuang H: Minimal lymphatic leakage in an infant with chylothorax detected by lymphoscintigraphy SPECT/CT. Pediatrics 2014; 134: e606–e610
12) Kinmonth JB: Lymphangiography in man: A method of outlining lymphatic trunks at operation. Clin Sci 1952; 11: 13–20
13) Kinmonth JB: A review of some technical points. Lymphology 1977; 10: 102–106
14) Itkin M, Kucharczuk JC, Kwak A, et al: Nonoperative thoracic duct embolization for traumatic thoracic duct leak: Experience in 109 patients. J Thorac Cardiovasc Surg 2010; 139: 584–589, discussion, 589–590
15) Dori Y, Zviman MM, Itkin M: Dynamic contrast-enhanced MR lymphangiography: feasibility study in swine. Radiology 2014; 273: 410–416
16) Chavhan GB, Amaral JG, Temple M, et al: MR Lymphangiography in children: Technique and potential applications. Radiographics 2017; 37: 1775–1790
17) Itkin M: Magnetic resonance lymphangiography and lymphatic embolization in the treatment of pulmonary complication of lymphatic malformation. Semin Intervent Radiol 2017; 34: 294–300
18) Itkin MG, McCormack FX, Dori Y: Diagnosis and treatment of lymphatic plastic bronchitis in adults using advanced lymphatic imaging and percutaneous embolization. Ann Am Thorac Soc 2016; 13: 1689–1696
19) Nowakowska D, Gaj Z, Grzesiak M, et al: Successful treatment of fetal bilateral primary chylothorax: Report of the two cases. Ginekol Pol 2014; 85: 708–712
20) Kato M, Nomura K, Ko Y, et al: The use of indocyanine green lymphography for the treatment of postoperative chylothorax with lipiodol lymphangiography in a 2-year-old child. J Pediatr Surg Case Rep 2017; 23: 46–49
21) Kirschen MP, Dori Y, Itkin M, et al: Cerebral lipiodol embolism after lymphatic embolization for plastic bronchitis. J Pedod 2016; 176: 200–203
22) Koshima I, Tashiro K, Kato M, et al: Surgeries for lymphedema. Rinsho Fujinka Sanka 2014; 68: 704–712 (in Japanese)
23) Kato M, Yamamoto T: Simple wire retractor for supermicrosurgical lymphaticovenular anastomosis. Microsurgery 2015; 35: 335–336
24) Kato M, Koshima I, et al: Surgical Treatments for Lyphedema. Tokyo, Person Shobo, 2017, pp 53-62
25) Kato M: Pediatric lymphatic diseases. Pediatric lymphatic surgery. J Clin Exp Med. 2017; 262: 1162–1166 (in Japanese)
26) Kato M, Watanabe S, Kato R, et al: Spontaneous regression of lymphangiomas in a single center over 34 years. PRS Global Open 2017; 5: e1501
27) Kato M, Watanabe S, Iida T, et al: Peri-orbital lymphangioma treated by lymphatic-venous anastomosis with indocyanine green lymphography analysis. J Pediatr Surg Case Rep 2017; 23: 9–14
28) Kato M, Watanabe S, Iida T: Diagnostic imaging for lymphatic disorders: Indocyanine green lymphangiography. Jpn J Ped Surg 2016; 48: 1270–1274
29) Kato M, Watanabe S, Iida T, et al: Venous anastomosis procedure for treatment of lymphatic malformation in Klippel-Trenaunay syndrome. J Pediatr Surg Case Rep 2017; 20: 1–3
30) Itkin M: Lymphatic intervention is a new frontier of IR. J Vasc Interv Radiol 2014; 25: 1404–1405
31) Nadolski GJ, Itkin M: Thoracic duct embolization for nontraumatic chylous effusion: Experience in 34 patients. Chest 2013; 143: 158–163
32) Dori Y, Keller MS, Rome JJ, et al: Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 2016; 133: 1160–1170