Present illness: A 4-year-old boy was taken to a regional medical center by his parents due to a 2-day history of stomach ache and a 1-day history of malaise. Blood tests, electrocardiography (ECG), and echocardiography led to a diagnosis of AMI. The patient was subsequently transferred and admitted to our institution more than 18 hours after the onset of stomach ache as the initial symptom.
Previous episodes: The patient had developed KD 2 months prior to the AMI event this time. Having suffered from fever initially, he saw a doctor at a local hospital on day 3. He was diagnosed with cervical lymphadenitis, because of bilateral cervical lymphadenopathy, and treated with ceftriaxone. The symptoms, however, did not improve. Bilateral conjunctivitis was noted on day 4 and erythema of the palms and soles, skin rash, strawberry tongue, and reddened lips developed on day 5. At that stage, he was diagnosed as KD. Coronary arterial lesions were absent on echocardiography. This patient was treated with IV high-dose immunoglobulin, IV prednisolone, and oral aspirin. His body temperature decreased once, but rose again on day 6. IV high-dose immunoglobulin was repeated on day 7. Despite these treatments, his body temperature did not normalize. Subsequently, cyclosporine A was administrated on day 8 and the symptoms improved. Echocardiography on day 10 showed bilateral coronary arterial lesions that worsened on day 12. With these findings, the patient was referred to the regional medical center. Although there were no longer symptoms of KD, the C-reactive protein (CRP) levels did not settle, and he was treated with infliximab. Dipyridamole was given as an antithrombotic agent. Echocardiography on day 14 showed giant coronary artery aneurysms and IV heparin was started. His body temperature rose again; plasmapheresis was carried out from day 17 to 20. After the treatment, fever or abnormal CRP did not recur. He was discharged on oral warfarin because of the giant coronary artery aneurysms that remained. Coronary angiography a month after the KD onset illustrated significant coronary artery aneurysms ranging from 4.0 to 12.9 mm in diameter (Fig. 1).
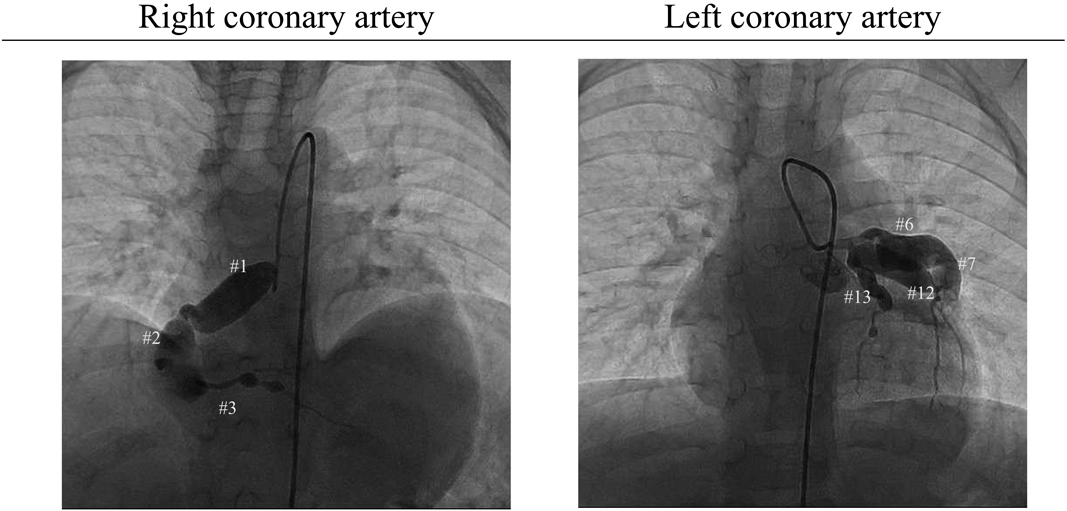
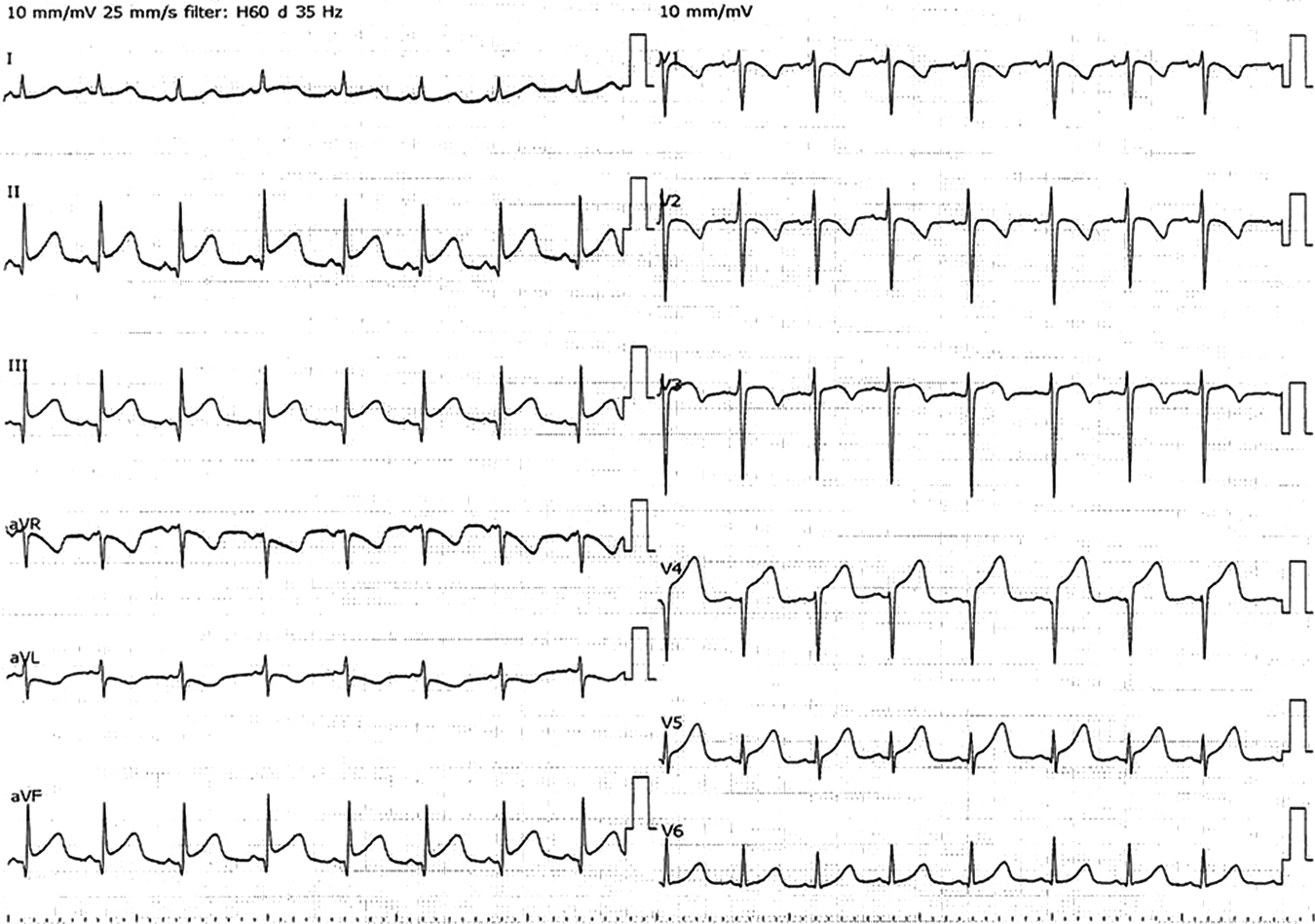
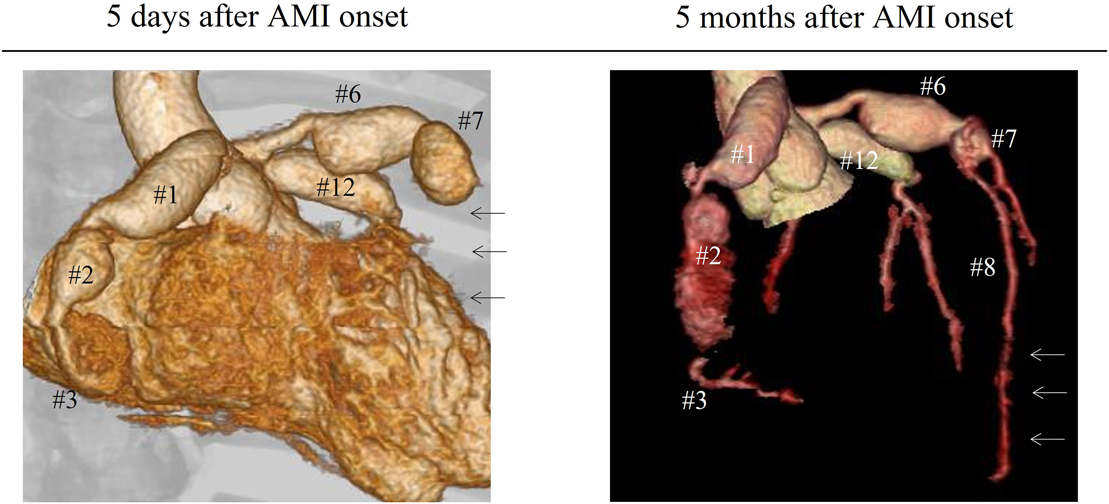
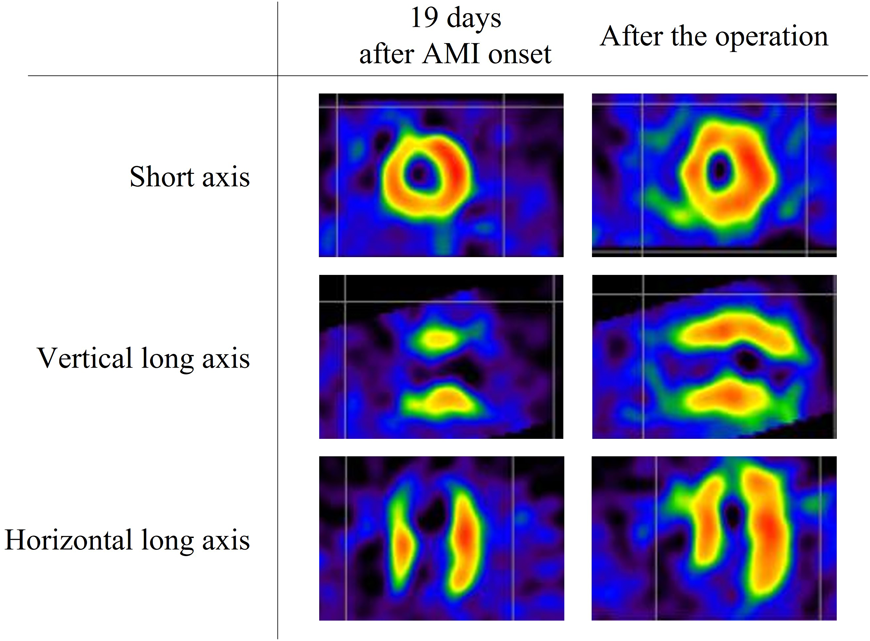
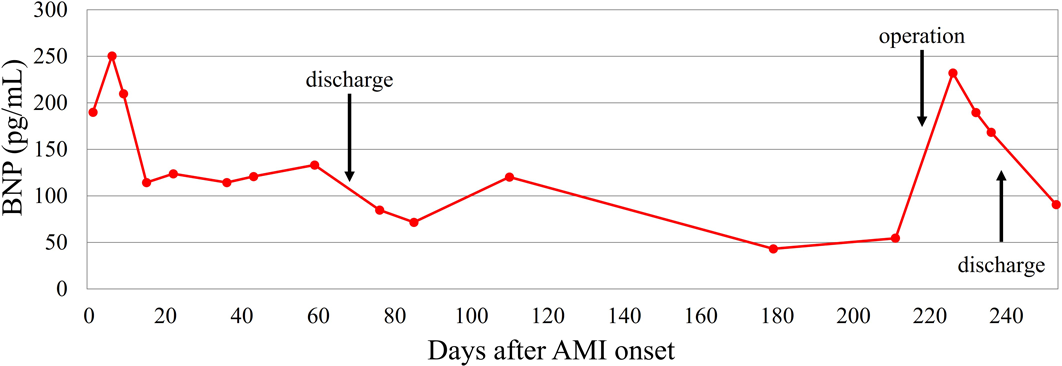
Admission to our institution this time (AMI): He had been on oral aspirin (85 mg), dipyridamole (50 mg), and warfarin (6.5 mg). The patient’s anthropometrics and vital signs were: height, 105.2 cm; weight, 18.2 kg; heart rate, 127 beats/min; blood pressure, 101/56 mmHg; and SpO2, 100%. There were no abnormal physical findings, except malaise. Blood tests revealed: creatine kinase, 1136 IU/L; creatine kinase MB, 118 IU/L; troponin I, 5000 pg/mL; brain natriuretic peptide (BNP), 52.3 pg/mL; and international normalized ratio of prothrombin time (PT-INR), 2.55. ECG demonstrated ST elevation in leads II, III, aVF, V3, and V4 (Fig. 2). Echocardiography showed dyskinesis of a part of the interventricular septum and the apex (Movie 1). After admission, he received continuous IV heparin infusion (the target value of activated partial thromboplastin time was twice the control). The patient was not eligible for primary percutaneous coronary intervention or thrombolytic therapy because more than 18 hours had elapsed since the onset of his symptoms and also he had received sufficient anticoagulant therapy.2) The stomach ache and malaise disappeared the following day. The left coronary artery was completely obstructed at the segment 7 on computed tomography (CT) and coronary angiography (Fig. 3, Movie 2). Myocardial perfusion scintigraphy revealed ischemia of the apex (Fig. 4). His warfarin dose required repeated adjustments to maintain the PT-INR at a desired level. He was finally discharged on day 68 after the admission for AMI.
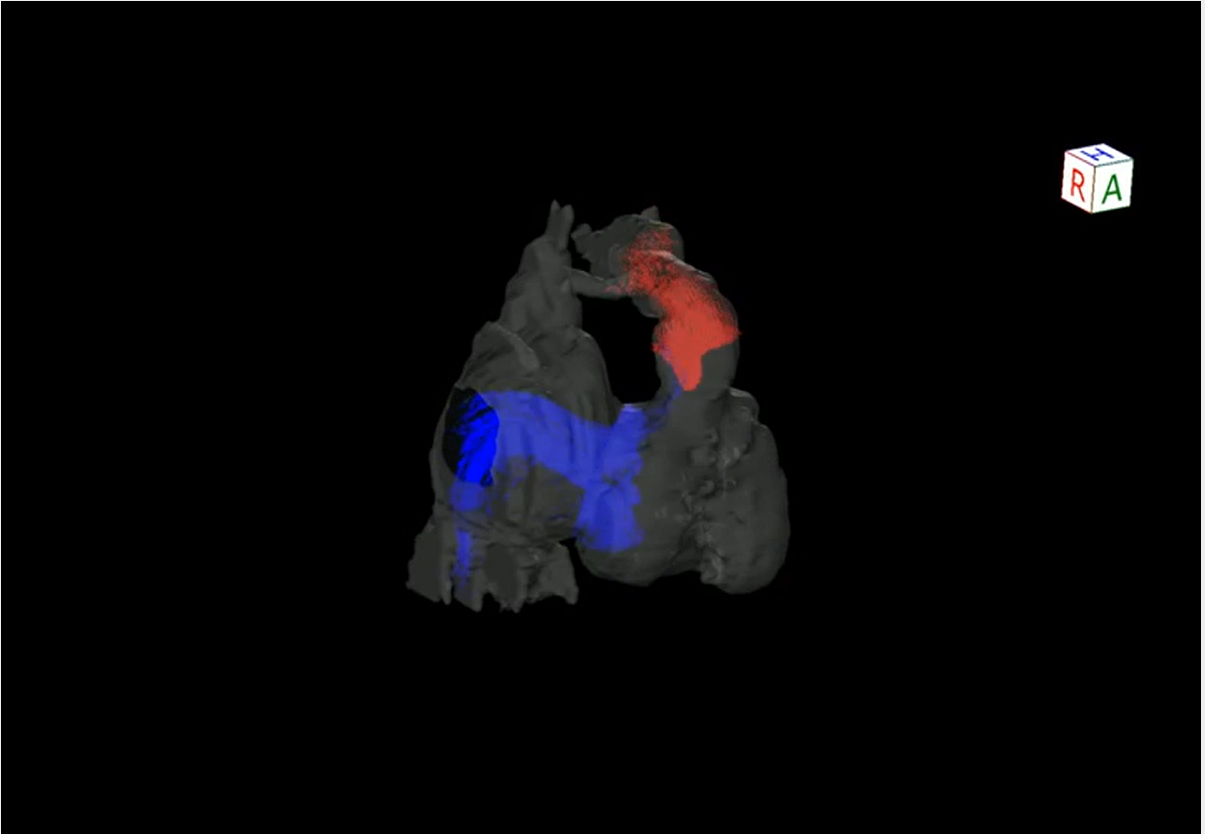
Following the AMI event: At a follow-up after discharge, echocardiography showed improved cardiac function and hypokinesis of the cardiac apex (improved from dyskinesis at AMI presentation) (Movie 1). Enhanced CT demonstrated recanalization of the obstruction at the segment 7 (Fig. 3). Moreover, the BNP values gradually decreased. We judged that the myocardium there remained partially viable (Fig. 5). We also considered that damage to the myocardium would be more significant if myocardial ischemia recurred, whereas cardiac function would improve still further if the blood flow was provided sufficiently. Six months after the AMI diagnosis, we carried out CABG using the left internal thoracic artery to the segment 8 to maintain the blood flow beyond the segment 7. The postoperative course was uneventful and myocardial perfusion scintigraphy demonstrated an improved perfusion of the apex (Fig. 4). He was discharged on the 16th postoperative day, on aspirin (100 mg), dipyridamole (50 mg), warfarin (5.7 mg), and enalapril (3.6 mg). His cardiac function improved further after the operation (Movie 1). On cardiovascular angiography 3 months after CABG, ejection fraction of the left ventricle remained impaired, while the cardiac index improved. Flow through the left internal thoracic arterial graft was smooth into the distal portion of the left anterior descending artery (Movie 2).
By the way, his elder brother also had KD at the age of 1 year, with no recurrence or long-term effects as seen in this patient.
Coronary arterial lesions represent the most significant long-term complication of KD due to an increased risk of AMI.1) Previous studies have shown that 0.02% of KD patients with giant aneurysms who were receiving warfarin developed AMI due to acute thrombotic obstruction; in most cases AMI occurring within 2 years of the KD onset.3, 4) The desired value of PT-INR in such cases is 2.0–2.5, and the optimal dose of warfarin considered for administration is 0.04–0.10 mg/kg. In our case, the patient developed AMI despite receiving two antiplatelet drugs and 0.36 mg/kg of warfarin. We suspected that he was less sensitive to warfarin, and that microthrombi had been formed prior to the AMI event.
Myocardial viability is a significant factor to consider in performing CABG, which includes the so-called ‘hibernating’ or ‘stunned’ myocardium. When blood flow declines, the hibernating myocardium resorts to reduced oxygen demand by decreasing contraction; this process is reversed upon reperfusion of the vessel.5) Myocardial perfusion scintigraphy using beta-methyl-p-[123I]-iodophenyl-pentadecanoic acid is useful in identifying hibernating myocardium.6) The stunned myocardium refers prolonged dysfunction after reperfusion.7) In our case, it was difficult to determine whether improvement of contraction at the apical area was associated with the hibernating phenomenon or the natural course of stunned myocardium, either during the follow-up or the postoperative period. In this respect, the particular myocardial perfusion scintigraph above-mentioned could have been informative. It remained unknown when recanalization of the coronary arterial obstruction actually occurred during the course in which cardiac function gradually improved as shown on echocardiography in the follow-up process. Regardless of the timing, we considered that the area was not entirely necrotic, which meant that myocardial viability was maintained, and performing CABG was necessary to avoid recurrent AMI to improve blood supply to the area. Our decision was sensible after all because cardiac function improved following the operation.
In conclusion, our patient developed AMI because of occlusion at the segment 7 subsequent to complicated KD. The dysfunctional area did not necrotize completely. We believe that aggressive CABG contributed to the improved prognosis of his cardiac function.