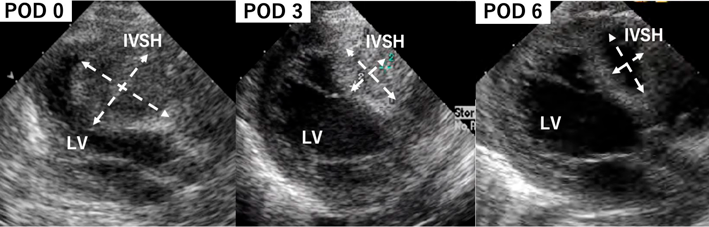
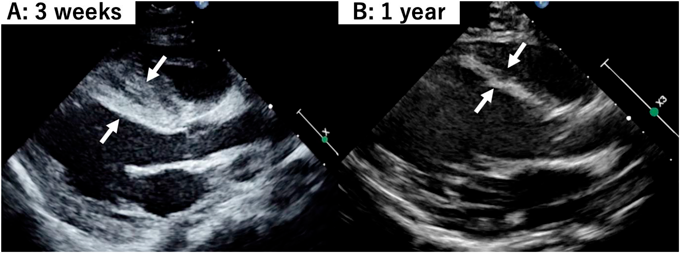
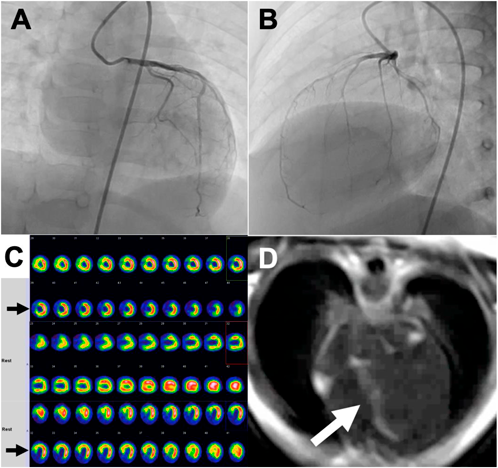
Atypical Ischemic Cardiomyopathy after Resolution of a Giant Interventricular Septal Hematoma after Repair of Ventricular Septal Defect
1 Department of Cardiovascular Surgery, Japan Community Health Care Organization (JCHO), Kyushu Hospital ◇ Fukuoka, Japan
2 Departments of Cardiovascular Surgery and Pediatric Cardiology, Japan Community Health Care Organization (JCHO), Kyushu Hospital ◇ Fukuoka, Japan