Congenitally corrected transposition of great arteries (ccTGA) is a rare congenital heart defect with a prevalence of less than 0.5%.1) Among surgical repair techniques, physiological repair leaves the morphologically right ventricle (RV) as a systemic ventricle. As a result, patients with physiologically repaired ccTGA carry risks of developing systemic RV failure and tricuspid valve regurgitation. To maintain the RV well-functioning with the tricuspid valve competent, cardiac resynchronization therapy and/or tricuspid valve plasty can be applied. Still, some patients may require placement of a ventricular assist device (VAD) and heart transplantation. VAD placement for the systemic RV poses technical challenges, especially in patients with mesocardia or dextrocardia.
Our patient is a 38-year-old female, weighting 53 kg with ccTGA and pulmonary stenosis. She had a surgical history of physiological repair at 10 years old, followed by closure of residual ventricular septal defect and plasty to the pulmonary artery at 19-years old. She also had a medical record of ventricular fibrillation at 27 years old, which required cardiopulmonary resuscitation and eventual cardiac resynchronization therapy. Due to gradual worsening of systemic RV function and tricuspid regurgitation over 10 years, she was listed for heart transplantation at the age of 37 years. She needed recurrent hospitalization for symptomatic heart failure and became inotropic dependent during this admission.
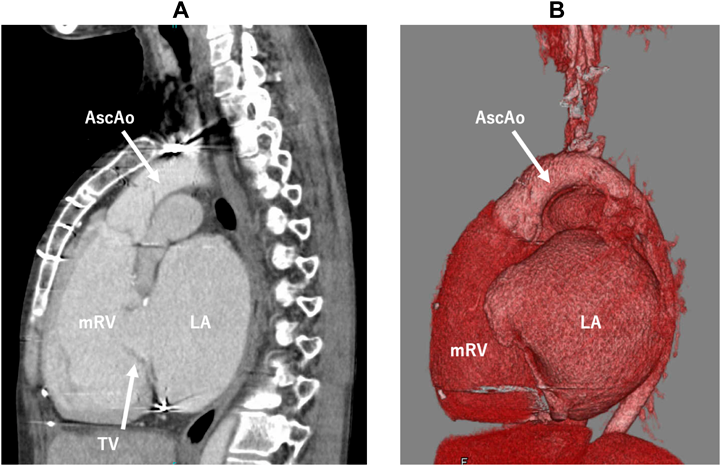
Her cardiac anatomy featured mesocardiac position, the systemic RV longitudinally elongated, and a short sagittal distance between the tricuspid valve and the apex of the systemic RV due to a severely dilated left atrium. The aorta was left anterior to the pulmonary trunk (Fig. 1). Therefore, standard VAD placement from the cardiac apex would cause troublesome sternal closure interfered by the pump body, inflow cannula obstruction due to close proximity to the tricuspid valve, and difficult anastomosis to the short anterior ascending aorta. In contrast, vertical VAD placement from the diaphragmatic surface towards the aortic valve seemed less hazardous resolving these challenges.
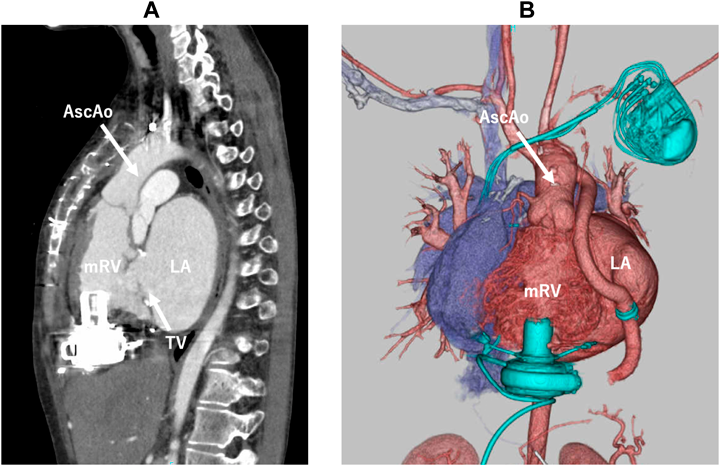
At the operation, a redo sternotomy was performed and a cardiopulmonary bypass was established through femoral cannulation. Under transesophageal echocardiogram guidance, the optimal inflow cannulation site was decided to avoid tricuspid tissue involvement. After aortic cross-clamping, trabeculation of the systemic RV was adequately resected and the HeartWare HVAD (Medtronic, Minneapolis, MN) was placed vertically from the RV diaphragmatic surface. The outflow graft was anastomosed to the left-lateral side of the ascending aorta. Postoperatively, the VAD flow had been stable with no evidence of tissue sucking or thrombus formation (Fig. 2). She was discharged home and waiting for heart transplantation as an outpatient.
Due to the increasing number of patients with adult congenital heart disease, more VAD implantation is to be expected in this population. Moreover, 34% of patients with ccTGA could develop heart failure in their 30 s2) and physiological repair has been associated with moderate to severe RV dysfunction in 36% of patients with 48% mortality at 30-year follow-up.3) In fact, ccTGA accounted for 36% of adult congenital heart disease patients in the INTERMACS database.4) Nonetheless, a standard implantation technique to systemic RV has not been established. Complex structures of the morphologically RV may hinder VAD flow, and a moderator band and abundant papillary muscles of the morphologically RV may increase the risk of pump thrombosis.5) Thus, careful muscular resection is required. In addition, since the shape of the RV and its apical position are various in ccTGA patients, it is crucial to determine the optimal inflow site in each case using transesophageal echocardiography with the heart beating. As for the outflow, a graft is laid along right atrium, in general, and anastomosed to right anterolateral aspect of the ascending aorta using a side-biting clamp under the beating heart condition. This standard design is not recommended in ccTGA patients, since the outflow graft should traverse the pulmonary artery before reaching the ascending aorta. In our present patient, therefore, vertical placement of the VAD via the diaphragmatic surface towards the aortic valve was applied in order to avoid inflow obstruction by the tricuspid valve tissue and to facilitate easier sternal closure. The outflow graft was placed left to the heart and anastomosed to the left lateral side of the ascending aorta which was short. This technique can be used in a similar way for any implantable device in ccTGA patients with a systemic RV.
The inflow cannula is short and the pump body is small with recent centrifugal VADs, these features allowing more options in either inflow or outflow arrangement.1, 5) It is important to determine how to place a VAD based on thorough understanding of unique characteristics of ccTGA so as to obtain sufficient result. The point was proven in our present patient.
Vertical placement of a VAD to the systemic RV is a good option in patients with failing systemic RV and mesocardia, as well as the outflow cannula anastomosing to the left side of the ascending aorta even though the structure is short.
Author Contribution
Hisashi Yoshida, Ryogo Hoki, Junko Katagiri and Takeshi Shinkawa conceived the idea of this implantation and contributed to the interpretation of data. Hisashi Yoshida drafted the original manuscript. Takeshi Shinkawa and Hiroshi Niinami supervised the conduct of this case. All authors reviewed the manuscript draft and revised. All authors approved the final version of the manuscript to be published.