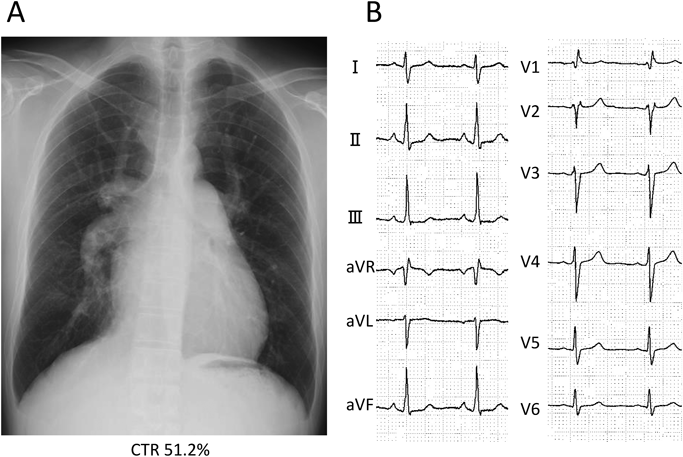
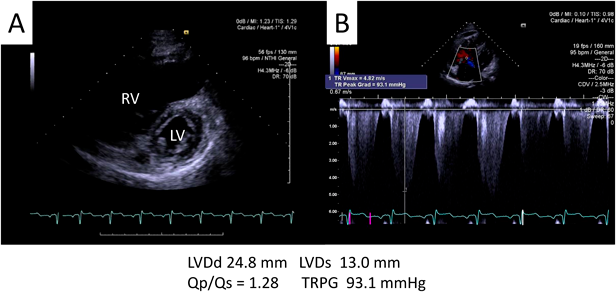
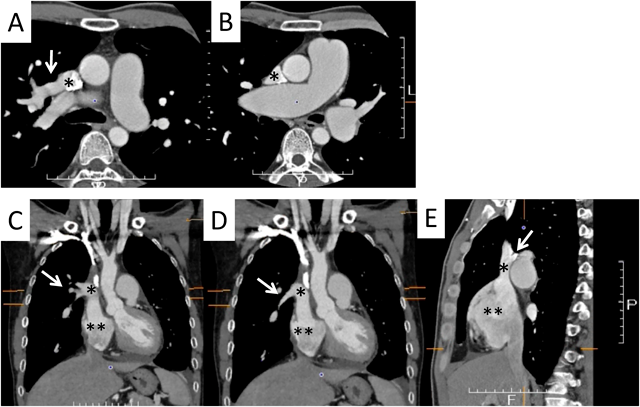
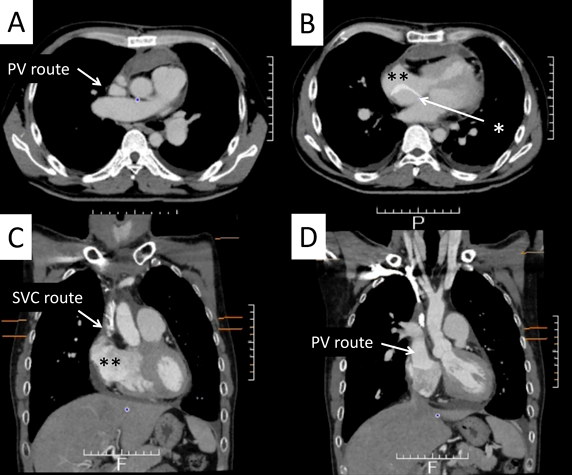
A 42-year-old man was referred to our hospital because of fatigue and exertional dyspnea; he often complained dyspnea when going up stairs. The cause of fatigue and dyspnea had not been detected, although he visited multiple clinics and his chest radiography at medical checkup already showed dilatation of the bilateral pulmonary arteries (Fig. 1A). He was unable to go to work due to his symptoms. On physical examination, his blood pressure and pulse rate were 114/86 mmHg and 78 bpm, respectively. He had a regular rhythm and a respiratory rate of 12 bpm with a percutaneous oxygen saturation of 98% in room air. His second heart sound was accentuated, and no heart murmur audible. A 6-minute walk distance was 390 m. These findings suggested that he was classified into New York Heart Association (NYHA) functional class III. Electrocardiography showed regular sinus rhythm, axis deviation and right ventricular hypertrophy with complete right bundle branch block (Fig. 1B). Echocardiography showed enlargement of the right ventricle and flattening of the interventricular septum. Doppler measurement of tricuspid regurgitation revealed that estimated right ventricular pressure was 103.1 mmHg (Fig. 2A, B). Contrast-enhanced computed tomography illustrated that the right upper and middle pulmonary veins (PVs) drained into the SVC, and that the pulmonary arteries as well as the right ventricle were dilated. Additionally, these anomalous PVs had been shifted upward due to the dilated right pulmonary artery (Fig. 3). During this investigation, the patient suddenly collapsed with low blood pressure, and immediate cardiopulmonary resuscitation was needed. Probably, PAH deteriorated subsequent to injection of contrast media, causing acute volume overload and an increase in pulmonary vascular resistance. Right heart catheterization (RHC) eventually revealed the systemic to pulmonary blood flow ratio (Qp/Qs) 1.4, mean pulmonary arterial pressure (PAP) 91 mmHg, pulmonary capillary wedge pressure 12 mmHg, and pulmonary vascular resistance (PVR) 20.3 WU, respectively (Table 1). Acute vasodilator test using the combination of inhaled oxygen and nitric oxide (iNO) showed PAP and PVR remaining unchanged. PAH related with heredity, drugs and left ventricular dysfunction, respiratory diseases were ruled out after PAH workup. Consequently, we started a combined therapy using PAH-specific drugs. Macitentan (20 mg/day) and tadalafil (10 mg/day) were immediately administered after the RHC. In addition, selexipag was gradually increased from 2 to 10 mg/day at our outpatient clinic. The second RHC took place 6 months after introduction of the PAH drugs, measuring mean PAP 49 mmHg (69/39 mmHg) and PVR 8.6 WU. The Qp/Qs ratio had increased to 2.0 (Table 1). The third RHC was carried out 9 months after initiation of the PAH drugs; mean PAP 45 mmHg (64/30 mmHg), which was similar to the value at the second RHC. Furthermore, PVR markedly improved (down to 2.7 WU), and the Qp/Qs ratio increased (up to 3.2) (Table 1). The patient’s exercise tolerance became better thereafter as his PAH improved. On the other hand, side effects of the PAH drugs, such as diarrhea and headache, gradually resulted in poor medication adherence. As interruption of PAH drugs likely results in progressive PAH, we decided to recommend surgical repair.
Table 1 Consecutive catheter data and use of agents treating pulmonary arterial hypertension | on admission | 6 months later | 9 months later | after surgery |
|---|
| RA a/v (mmHg) | 15/14 | 8/6 (5) | 12/9 (8) | 13/10 (9) |
| RV (mmHg) | 125/EDP 14 | 64/EDP 2 | 65/EDP 10 | 46/EDP 5 |
| PA (mmHg) | 126/69 (91) | 69/39 (49) | 64/30 (45) | 46/26 (34) |
| Pcwp (mmHg) | 13/13 (12) | 8/9 (7) | 21/19 (15) | 20/18 (15) |
| LV (mmHg) | 146/EDP 11 | 149/EDP 4 | — | — |
| Ao (mmHg) | 145/109 (126) | 135/82 (107) | — | — |
| PVR (WU) | 20.3 | 8.6 | 2.7 | — |
| Qp/Qs | 1.4 | 2.0 | 3.2 | — |
| PAH specific drugs |
|---|
| Tadalafil (mg) | — | 40 | 40 | 40 |
| Macitentan (mg) | — | 10 | 10 | 10 |
| Selexipag (mg) | — | 0.4 → 0.8 → 3.2 | 3.2 | 3.2 |
| Ao, aorta; EDP, end diastolic pressure; LV, left ventricle; PA, pulmonary artery; Pcwp, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; Qp/Qs, pulmonary to systemic blood flow ratio; RA, right atrium; RV, right ventricle; WU, Wood unit. |
Operative Findings
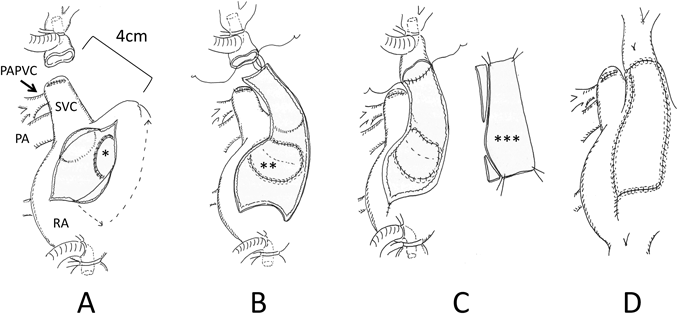
Surgery was carried out through a median sternotomy. At first, the superior vena cava (SVC) was extensively dissected, and then cardiopulmonary bypass was established inserting cannulae into the ascending aorta and the caval veins. The cannula into the SVC was placed as high as possible distant from the right atrium (RA), and a venting tube was inserted into the main pulmonary trunk. The SVC was divided just above the right anomalous PVs, and the proximal stump of the SVC was directly closed. The RA appendage could not be mobilized to the distal end of the SVC for direct anastomosis, because the RA appendage was less flexible due to severe RA dilatation and thickening of its wall. There was a distance of 4 cm between the distal stump of the SVC and the RA appendage (Fig. 4A). After inducing cardiac arrest, the RA was incised. The atrial septum was incised at the fossa ovalis, and an ASD, approximately a diameter of 15 mm, was created surgically (Fig. 4A). The edge of the created ASD was sewed carefully by means of the so-called intima-to-intima suturing in order to maintain continuation of the endocardial surface (Fig. 4A). The right anomalous PVs were rerouted into the left atrium through the ASD using an extended polytetrafluoroethylene prosthetic patch (Fig. 4B). After releasing the aortic clamp, heart rhythm recovered in sinus rhythm. The posterior wall of a neo-SVC channel was reconstructed using an atrial wall flap that was created from the anterior wall of the RA. The anterior wall of the neo-SVC was augmented using a fresh autologous pericardial patch (Fig. 4B, C, D). Weaning from cardiopulmonary bypass was successful with iNO. Arterial blood pressure was 83/51 mmHg, and PAP was 38/23 mmHg. The durations of the operation, cardiopulmonary bypass, and aortic cross-clamp were 260, 149, and 68 minutes, respectively.
Postoperative Course
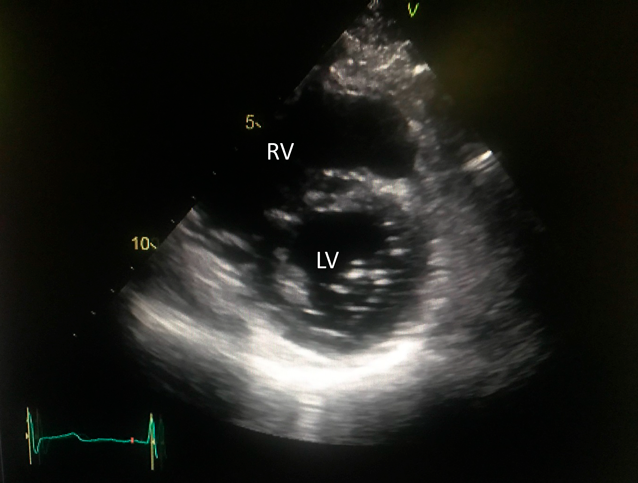
The PAH drugs were resumed, in the same doses as preoperatively administered, via a gastric tube from the first postoperative day. After iNO ceased, the patient was weaned off mechanical ventilation on the second postoperative day. On the following day, oral intake was restarted, and oral warfarin was prescribed for anticoagulation therapy. Inotropic support was gradually decreased and stopped on the 5th postoperative day. On the 6th postoperative day, nasal oxygen cannula was removed, and he was discharged from the intensive care unit to a general ward. Postoperative enhanced CT revealed that the neo-SVC and the PV routes had no obstruction (Fig. 5). Electrocardiogram showed sinus rhythm. Transthoracic echocardiography suggested that blood flows across the right PVs and the SVC had been well established, and that PAH had improved (Fig. 6), Thereby the patient was discharged home on the 16th postoperative day. His clinical status improved to NYHA functional class I. He returned to his work at 2 months after surgery. The dosage of selexipag was gradually weaned according to the echocardiographic findings. We performed RHC at 15 months after surgery, which showed that mean PAP decreased to 34 mmHg. Macitentan and tadalafil were continued although selexipag was changed to beraprost due to adverse effects such as diarrhea and face flushing.
Pathophysiology of PAH in Adults With Isolated PAPVC
The most common type of PAPVC is a right upper PV draining to the SVC, followed by the right PVs directly to the RA or the left PV to the left innominate vein.1) It is not yet clear which types of PAPVC tend to lead to PAH, since this malformation is relatively rare and data regarding the PAH is limited. Isolated PAPVC is a quite unusual form of CHD1, 2) that could cause PAH in adults.3) Majadalary et al.4) reported patients with two or more anomalous PVs were likely to have right ventricular enlargement requiring intervention. Some patients develop severe PAH even with a solitary anomalous PV, since the amount of anomalous venous flow in isolated PAPVC directly overload the amount of pulmonary arterial flow. Therefore, we need to follow carefully the clinical course in patients with isolated PAPVC regardless of the number of abnormal PVs.
Assessment and Treatment for CHD With PAH
A patient with CHD and PAH should undergo surgical treatment when pulmonary vascular resistance is low, particularly below 3 WU.5) In contrast, a therapeutic strategy in adults with isolated PAPVC and significant PAH remains unclear. In our patient, initial PVR was extremely high (over 20 WU). With this finding, it was judged that repair of an intra-cardiac shunt was infeasible based on the guidelines for adult ASD closure by European Society of Cardiology and European Respiratory Society (ESC/ERS).5) Closure of a shunt across an ASD may be indicated when PVR 5 WU or greater declines to less than 5 WU with PAH treatment as far as ordinary ASD physiology is concerned. In our present patient, testing for acute vaso-reactivity with iNO showed negative response. We challenged further to assess reversibility and operability after using multi-pathway medication (Endothelin, NO or Prostacyclin) with a wish for improvement. We therefore readily proceeded to upfront combination therapy with three classes of specific PAH medication. Over the course of the PAH therapy, the patient’s cardiac output gradually increased with PVR decreasing, and his exercise tolerance significantly improved. In recent years, the so-called “treat-and-repair strategy” has been attracting attention as a brand-new approach.6) Closure of intra-cardiac shunts is to be established after PAH control using specific drugs for CHD with PAH. There are only a few reports about “treat and repair technique” for isolated PAPVC with severe PAH.6–8) As for adult ASD patients with PAH, some criteria have been proposed in Japan based on clinical results of PAH treatment using multiple medications. Kijima et al.7) reported that the criteria for percutaneous ASD closure would be PVR <8 WU and Qp/Qs ≥1.5 after PAH treatment. Yao et al.8) suggested that the criteria for managing ASD closure should be a PVR of <7.5 WU, a Qp/Qs of >1.5, and right ventricular ejection fraction of >0.4 after PAH treatment. These criteria were based on studies with a relatively small cohort, and details of postoperative administration of PAH drugs remained unclear. In our PAPVC patient, the RHC 9 months after initiation of upfront combination therapy revealed a drastic decrease of PVR (down to 2.7 WU). Without a radical repair, the patient’s PAH could return to a difficult circumstance because of poor medication adherence due to adverse effects. We were concerned about it. This was the reason we concluded that his intra-cardiac shunt should be repaired on the basis of the above-mentioned criteria for ASD closure. We discussed whether leaving a small ASD fenestration would be necessary; the hole could have acted as a safety-valve for postoperative PAH crisis. We decided, nonetheless, not to provide such a residual communication; we regarded it unnecessary considering his low PVR.
Surgical Technique for Isolated PAPVC
A couple of crucial points at surgery for PAPVC repair are how to protect sinus nodal function and how to prevent obstruction across the SVC and the PV routes. The surgical procedures are affected by how high the anomalous PVs are connected to the SVC, how large the diameter of the SVC is, and how large the volume of the RA is. Said et al.9) recommended the use of the Warden procedure with superior caval division for PAPVC repair when an anomalous PV drained into the SVC more than 1 cm above the cavo-atrial junction. When direct anastomosis turned out to be difficult between the distal stump of the SVC and the RA appendage, a Gore-Tex tube graft was interposed as a modified Warden procedure. We suggest that the caval division technique should be accompanied with adequate mobilization of the cephalad part of the SVC and the RA appendage. The anomalous PV connection could have been shifted upward due to dilatation of the right PA. In our patient, the anomalous PVs drained into a quite distal segment of the SVC (Fig. 4A). We therefore chose the caval division procedure. As described above, a flap made of the anterior wall of the RA was used for reconstructing the posterior wall of the new SVC channel.10) The anterior wall of the channel was augmented using a fresh autologous pericardial patch. Thus, the new SVC pathway is surrounded by autologous tissues. These maneuvers were carried out after intra-atrial rerouting, evaluating the distance between the distal stump of the divided SVC and the RA, and creating a suitable size of the RA wall flap after removal of the aortic cross-clamp. This is a useful alternative surgical method to treat adult patients whose anomalous PVs returns high of the SVC. In addition, this method was safer and technically easier than other previous techniques. Both routes continued to provide smooth flows at the 1-year follow up.
Diagnosis of CHD in Adulthood
Idiopathic PAH diagnosed in adulthood might include operable CHD once PAH is controlled using specific drugs. Thus, CHD should be sought in adult cases with idiopathic PAH, and PAH treatment should be initiated promptly after the diagnosis. The contrast-enhanced CT is useful for diagnosing PV anomalies,2) but use of contrast media in CHD patients with PAH might worsen the degree of PAH. Especially, such an adverse effect in a CHD patient without intra-cardiac shunt is similar to that in idiopathic PAH patients. The contrast-enhanced CT should be performed after meticulous explanation about the risk of reactive PAH inducing cardiogenic shock in patients with severe PAH. In addition, PAH treatment should be continuously administered for potential PAH after surgery. In patients with PAH after repair, PAH could be either still present immediately after repair of an intra-cardiac shunt or could re-deteriorate several months or years after the procedure.11) Integrated treatments consisting of preoperative PAH treatment, intra-cardiac repair and postoperative remedy for residual PAH might contribute to improving the quality of life in the group of CHD with PAH. Unfortunately, it was unable to identify surgical criteria or evidences for this approach in prospective studies previously reported for unrepaired adult patients with CHD, PAH and intra-cardiac shunts due to a limited patients’ population. In this regard, the accumulation of relevant data on treatments for PAH in adult CHD patients would serve to establish new guidelines. Further studies are required to investigate the long-term results after PAPVC repair in adulthood.