Transposition of the great arteries (TGA) associated with a borderline right ventricle (RV), ventricular septal defect (VSD), and aortic arch obstruction presents a clinical challenge. Because of pulmonary over-circulation, palliation to restrict pulmonary blood flow is required early during the neonatal period. Whether biventricular repair is feasible or not remains uncertain at this stage. Palliative arterial switch operation (ASO) has been reported as an alternative to the Norwood or Damus-Kaye-Stansel procedure in patients with single-ventricular anatomy, TGA, and subaortic obstruction.1–3) As opposed to the Norwood or Damus-Kaye-Stansel procedure, the palliative ASO provides antegrade pulmonary blood flow from the RV, preserving the potential for biventricular repair. After such palliative ASO, biventricular repair would turn out to be feasible when size of tricuspid valve (TV) and RV function are favorable. One and a half ventricular repair (1.5VR) is an alternative surgical option for borderline RV. This unique pattern of circulation may result in a better circulatory status than the Fontan circulation, while superior vena cava (SVC) hypertension is a recognized complication.4, 5)
Herein, we describe a patient with TGA, borderline RV, VSD, and coarctation of the aorta, who underwent the ASO with atrial septal defect (ASD) open to determine the potential of biventricular repair.
Prior to Palliative ASO
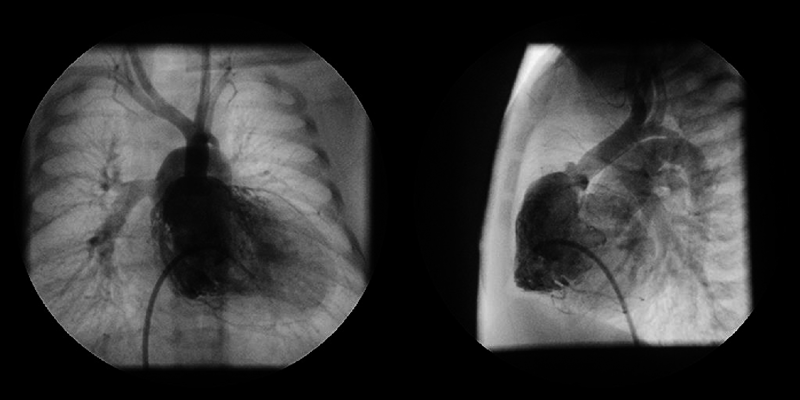
A 0-day-old boy was transferred to our hospital because of cyanosis and a heart murmur. On arrival, two-dimensional echocardiography revealed TGA, VSD, coarctation of the aorta, and a moderately hypoplastic RV with tripartite portion and a TV diameter of 7.5 mm; Z-score (the distance of the raw score value from the mean in terms of standard deviation) being −1.62. Anatomy of the coronary artery was of a usual pattern (the left anterior descending branch and the circumflex artery arising from sinus 1 and the right coronary artery from sinus 2). VSD was of a perimembranous type. RV angiography on Day 2 is shown in Fig. 1. The aortic valve diameter was 6.7 mm (Z-score: 0.14) without subaortic stenosis. Infusion of prostaglandin E1 (PGE1) was immediately initiated, but the patient developed circulatory failure due to obstruction across the ductus arteriosus. At 5 days of age, urgent bilateral pulmonary artery (PA) banding was performed; the patient was then stabilized on continuous PGE1 infusion.
Palliative ASO
The relative size of the TV remained unchanged, with a Z-score of −1.66. At 2 months of age, the patient underwent the ASO with VSD closure and coarctation repair, while ASD was left open. Through a median sternotomy, mildly hypothermic standard bicaval cardiopulmonary bypass was established using a 3 mm Gore-Tex (W.L. Gore & Associates, USA) tube anastomosed to the brachiocephalic artery for aortic cannulation. The PA banding tapes were removed. The ductus arteriosus was resected and coarctation repair was performed in an extended end-to-end fashion. Under cardioplegic arrest, the ASO was performed employing the Lecompte maneuver. The coronary arteries were reimplanted with the trap-door technique. The PA was reconstructed using the autologous pericardium. The neo-aortic root was plicated to deal with size discrepancy between the ascending aorta and the neo-aortic root. VSD was closed using a Gore-Tex patch. Intraoperatively, the diameter of the TV annulus was 8 mm (Z-score: −2.3) and that of ASD 10 mm. After weaning from cardiopulmonary bypass, the patient showed severe cyanosis and a 3 mm shunt from the brachiocephalic artery to the PA was constructed to augment PA blood flow. Cardiopulmonary bypass time and cross-clamp time were 570 min and 155 min, respectively. The patient was transferred to intensive care unit with sternal splintage. The chest was closed on the 4th postoperative day and the patient was extubated on the 6th postoperative day. During the postoperative period, no further intervention was required, and oxygen saturation was maintained above 87% on room air.
After Palliative ASO
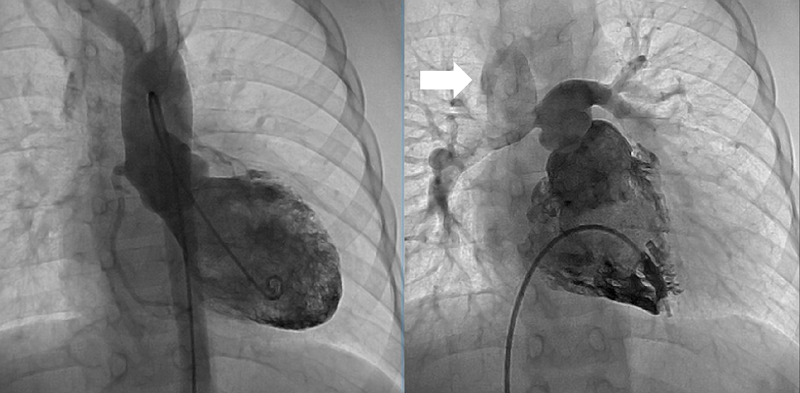
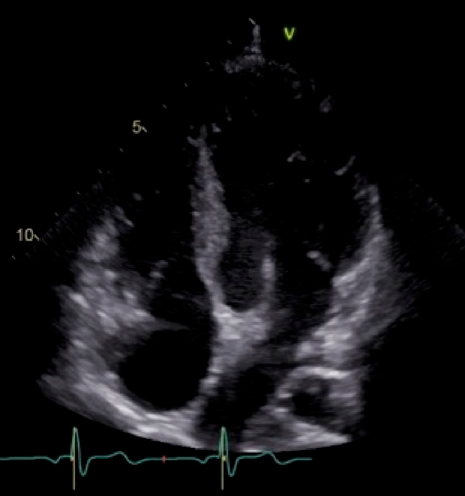
Two years after the operation, the diameter of the TV annulus was found to be 10.8 mm (Z-score: −2.73), and bidirectional flow through ASD was observed on echocardiography. Computed tomography showed that the systemic-to-pulmonary shunt had been occluded, suggesting increased antegrade pulmonary blood flow from the RV. Cardiac catheterization showed a mean PA pressure of 13 mmHg. At the age of 3 years and 6 months, 1.5VR was performed, consisting of the bidirectional Glenn anastomosis, ASD closure, and division of the systemic-to-pulmonary shunt already occluded. The diameter of the TV annulus was measured intraoperatively as 13 mm (Z-score: −2.03). Cardiopulmonary bypass time and cross-clamp time were 174 min and 22 min, respectively. Angiography at 1 year postoperatively showed adequate development of the PAs and unobstructed left ventricular outflow tract (Fig. 2). The SVC and right atrial pressures were 13 mmHg and 6 mmHg, respectively. Thirteen years after the operation, the patient is in a good general condition with normal sinus rhythm. The latest investigation on echocardiography illustrated no sign of right heart failure, such as dilatation of the inferior vena cava, a reversal flow in the inferior vena cava, and right ventricular systolic dysfunction. The TV diameter was 25.3 mm with balanced left and right ventricular cavities (Fig. 3). Table 1 summarizes consecutive changes in the TV diameter, a TV-to-mitral valve diameter ratio, mean PA pressure, and RV end-diastolic pressure. During the course, the patient did not show significant RV diastolic or systolic dysfunction. The TV diameter and RV function did not correlate to each other.
Table 1 Echocardiographic and cardiac catheterization data | Age (year) | BW (kg) | TV Z-score | TV/MV | Mean PAP (mmHg) | RVEDP (mmHg) |
|---|
| At birth | 0 | 2.4 | −1.62 | 0.70 | N/A | 6 |
| Prior to ASO | 0 | 3.1 | −1.66 | 0.63 | N/A | 6 |
| Prior to 1.5 VR | 2 | 11 | −2.73 | 0.66 | 15 | 8 |
| 1 year after 1.5 VR | 4 | 15 | −1.91 | 0.63 | 15 | 6 |
| Latest follow-up | 16 | 51 | −0.7 | 0.80 | N/A | N/A |
| 1.5 VR, one and a half ventricular repair; ASO, arterial switch operation; BW, body weight; MV, mitral valve; PAP, pulmonary arterial pressure; RVEDP, right ventricular end-diastolic pressure; TV, tricuspid valve; N/A, not applicable. |
Palliative ASO
The palliative ASO was initially reported by Freedom et al. as a part of the Fontan-Bjork operation (Anastomosis between the right atrium and the right ventricle, using the hypoplastic right ventricle as a pumping chamber) for tricuspid atresia and TGA.1) Following this report, several authors reported the usefulness of this procedure in functional single ventricle with TGA and subaortic stenosis.2, 3) Currently, the palliative ASO is considered an alternative approach to the Damus-Kaye-Stansel procedure or the Norwood operation (anastomosing the PA and the ascending aorta, using the right ventricle as a main pumping chamber in hypoplastic left heart syndrome) in this patient group. The palliative ASO is theoretically advantageous because subaortic stenosis disappears, a shunt-dependent pulmonary circulation is avoided, left PA stenosis is less likely, and the pulmonary vascular bed is naturally protected.
The present patient with TGA, small RV, VSD, and coarctation of the aorta has a small subaortic chamber. Blood inflow to the chamber is partially through the bulvo-ventricular foramen. This is a morphologic feature that may potentially lead to systemic ventricular outflow obstruction after volume-reducing palliation. Therefore, we discussed the utility of ASO in this patient. The potential option as the second surgical palliation following bilateral PA banding included; (1) coarctation repair and revision of banding onto the PA trunk, (2) the Damus-Kaye-Stansel procedure with coarctation repair and construction of a systemic-to-PA shunt, and (3) the palliative ASO and coarctation repair. In early 2000s, Serraf et al. reported the utility of a staged approach with coarctation repair and PA banding for TGA with hypoplastic RV and coarctation of the aorta.6) They reported a successful biventricular repair afterwards with the growth of right heart structures after coarctation repair and PA banding. In contrast, as several authors pointed out, PA banding in patients with a small subaortic chamber is not recommended because it may accelerate subaortic stenosis and subsequently ventricular hypertrophy.7, 8) Moreover, it should be noted that the median tricuspid Z-score in Serraf’s report was −0.9, which was larger than that of our case. The second option above mentioned can avoid subaortic stenosis and protect the pulmonary vascular bed. The drawbacks of this procedure are, however, shunt-dependent PA blood flow, high risk of left PA stenosis, and the greatly reduced potential of biventricular repair. In this regard, the ASO is a reasonable option to obtain concordant ventriculoarterial connections, maintaining the feasibility of biventricular repair in our case. The term “palliative arterial switch operation” has been used for ASO in single-ventricular physiology such as tricuspid atresia or double inlet left ventricle associated with ventriculo-arterial discordance. Therefore, palliative ASO usually means ASO with leaving VSD open. In our case, an ASO was performed in a patient with TGA and hypoplastic RV who could potentially undergo biventricular repair. Because of the small size of the TV, we closed VSD and left ASD open, as Serraf et al. described in their report.
Intentional ASD restriction has been reported to promote ventricular growth in patients with borderline LV.9, 10) The Boston group reported that fenestrated ASD closure with a 4 mm central fenestration provides an acceptable outcome in patients with measured atrioventricular valve Z-score less than −2.0,10) while atrial hypertension and elevated ventricular diastolic pressure are concerns, This concept could have been applied to the present case, anticipating promotion of RV growth and potentially eliminating the need for construction of a systemic-to-pulmonary shunt.
The optimal timing for the palliative ASO in our present patient remains to be clarified. Yerebakan et al. reported that hybrid stage 1 procedure can allow time for potential growth of the LV in patients with HLHS and its variants, avoiding major surgeries in the neonatal period.11) This strategy was successfully applied to the present patient, but the relative size of the TV did not change preferably. We eventually chose the palliative ASO at 2 months of age.
One and a Half Ventricular Repair
The outcomes after 1.5VR in patients with a hypoplastic subpulmonary ventricle have been widely investigated, with a 10-year transplant-free survival rate of 81.2–92.4%.12, 13) The idea of 1.5VR is to provide kinetic energy and pulsatility to the pulmonary blood flow while reducing the volume load to the RV. As for patient selection, Chowdhury et al. asserted that a moderately hypoplastic RV with a TV Z-score between −1.5 and −4.8 and without residual pulmonary hypertension may be appropriate for 1.5VR.13) This unique circulation may have some advantages over the Fontan circulation by recruiting the hypoplastic RV as a pumping chamber for the pulmonary circulation, while there are studies reporting that additional pulsatile pulmonary blood flow is at a risk of SVC hypertension, SVC aneurysms, pleural effusions, and chylothorax.4, 5) Prassana et al. reported that 39% of patients developed SVC hypertension during follow up after 1.5VR. They took down the Glenn anastomosis to establish the biventricular circulation in 5 patients, and 3 of them had SVC hypertension resolved.12) Our patient had growth of the right heart compartment after 1.5VR, and revision to biventricular physiology seems feasible. We are planning cardiac catheterization and cardiac MRI in this patient to determine whether biventricular conversion is indicated or not. Prasad et al. recently reported their experience of surgical conversion from a single-ventricular circulation to one and a half ventricular or biventricular physiology in patients with hypoplastic right ventricle.14) Based on cardiac MRI, the minimum RV end-diastolic volume for a successful biventricular circulation was 27 mL/m2 in pulmonary atresia with intact ventricular septum, and 22 mL/m2 in unbalanced atrioventricular septal defect with left ventricular dominance. A smaller RV/LV stroke ratio and a lower RV ejection fraction were factors significantly associated with failed biventricular circulation. Since the patient is in a good general condition with current 1.5VR circulation, it should be carefully determined whether the benefit of biventricular circulation surpass the risks of the fourth time operation.
Limitation
The significance of case report is limited by lack of ability to generalize and no possibility to establish cause-effect relationship. This case report demonstrated utility of the palliative ASO to provide time to determine the feasibility of biventricular repair for TGA with hypoplastic RV. We believe that this report will serve as an opportunity to gather attention to this option, which will enable clinical research using multiple patients. The outcomes of the palliative ASO for TGA with borderline RV need to be determined in further studies with a cohort of a sufficient number of patients.