An 11-year-old girl presented with fever, sore throat, and cough. Polymerase Chain Reacton (PCR) analysis using a specimen obtained from a nasal swab confirmed a diagnosis of COVID-19. SARS-CoV-2 sequencing was not performed, but the Omicron species was likely the variant because the patient presented in the early phase of the prevalence of this variant. Fever disappeared on the second day after the onset, while she started to feel mild chest pain on the third day which became worse over the following days. On the fifth day, she visited an emergency clinic with severe chest pain. On echocardiography, she had severe left ventricular (LV) dysfunction with an ejection fraction of 33.5% and moderate pericardial effusion. She was admitted to the intensive care unit of our hospital.
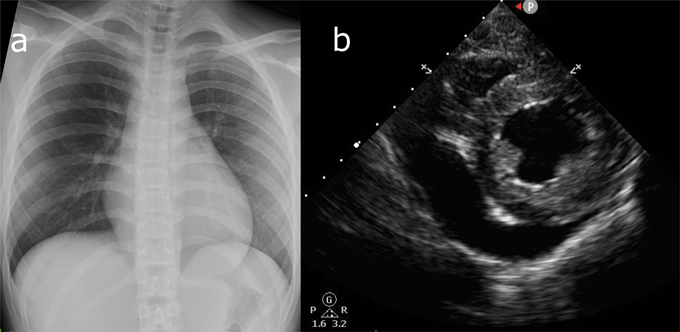
Her body temperature was 36.5°C, blood pressure 90/54 mmHg, heart rate 120/min, respiratory rate 28/min, and SpO2 100% in room air. A gallop rhythm was audible on auscultation of the heart, and extremities were cold. There was no hepatomegaly. A chest X-ray revealed no pneumonia or prominent cardiac enlargement (Fig. 1a). A blood test showed: white blood cell count 7,500/µL (neutrophil 78.5%, lymphocyte 18.6%, monocyte 2.8%), C-reactive protein 0.15 mg/dL, creatinine 1.02 mg/mL, creatine kinase (CK) 121 U/L, CK-myocardial band (MB) 4 U/L, and troponin-T 0.03 ng/mL, these suggesting myocardial damage and mild renal dysfunction.
Dopamine (3 µg/kg/min) and milrinone (0.3 µg/kg/min) were introduced for LV dysfunction, and intravenous immunoglobulin (1.5 g/kg in total over 4 days), dexamethasone (0.1 mg/kg/day for 4 days), and remdesivir (100 mg/day for 4 days) were also administered. Six hours after admission, her blood pressure decreased to 80/60 mmHg. Echocardiography showed a considerable increase in pericardial effusion (Fig. 1b). Surgical drainage of the effusion was performed, and biochemical analysis revealed total protein of 5.9 g/dL, albumin of 3.8 g/dL, and lactate dehydrogenase of 226 U/L, suggesting that the fluid was of an exudative nature. No cellular components were found in the pericardial fluid. On the sixth day, her body temperature rose to 39.0°C, and C-reactive protein (0.95 mg/dL), CK (1768 U/L), and CK-MB (37 U/L) had peaked to their highest levels of the illness course. After the seventh day, no febrile episodes were noted, and the LV ejection fraction gradually improved to 57.1% by the ninth day. The patient returned home in a healthy condition on the 20th day.
Table 1 shows the data of 27 cytokines and chemokines analyzed using the Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA). Cytokine concentrations in the pericardial fluid were markedly elevated compared with those in the serum, especially interleukin (IL)-6. IL-6 was 2344.1 pg/mL in the pericardial fluid and 6.13 pg/mL in the serum; the latter being within the reference range.
Table 1 Cytokine levels in serum and in pericardial fluid| Cytokine (pg/mL) | Serum (reference) | Pericardial fluid | Ratio (Pericardial/plasma) |
|---|
| IL-1β | 0.67 (0.52±0.27) | 1.62 | 2.41 |
| IL-1ra | 7657.75 (398.97±171.51) | 725.11 | 0.09 |
| IL-2 | 3.07 (6.68±2.83) | 13.41 | 4.37 |
| IL-4 | 1.82 (2.61±0.6) | 16.19 | 8.90 |
| IL-5 | 7.53 (8.23±1.71) | 69.13 | 9.18 |
| IL-6 | 6.13 (5.56±2.47) | 2344.09 | 382 |
| IL-7 | 46.88 (51.25±4.06) | 304.88 | 6.50 |
| IL-8 | 171.95 (75.99±45.12) | 312.89 | 1.82 |
| IL-9 | 198.45 (262.16±26.63) | 81.78 | 0.41 |
| IL-10 | 4.1 (5.44±0.73) | 27.3 | 6.7 |
| IL-12 | 1.6 (0.56±0.23) | 2.72 | 1.70 |
| IL-13 | 1.26 (2.13±0.51) | 6.91 | 5.48 |
| IL-15 | 129.34 (170.05±45.73) | 276.67 | 2.14 |
| IL-17 | 5.35 (21.31±9.76) | 47.23 | 8.83 |
| Eotaxin | 27.54 (50.04±7.68) | 432.46 | 15.7 |
| FGF basic | 13.5 (17.01±2.6) | 43.38 | 3.21 |
| G-CSF | 105.6 (796.77±438.52) | 296.3 | 2.81 |
| GM-CSF | 0.86 (1.97±0.72) | 7.21 | 8.38 |
| IFN-γ | 17.01 (3.6±0.94) | 90.89 | 5.34 |
| IP-10 | 2131.5 (1251.72±1254.61) | 39626.02 | 18.6 |
| MCP-1 | 24.71 (39.53±12.85) | 1431.32 | 57.9 |
| MIP-1α | 4.62 (56.26±34.61) | 2.04 | 0.44 |
| PDGF-bb | 3182.37 (5276.75±2230.68) | OOR below | — |
| MIP-1β | 134.8 (209.77±39.33) | 27.43 | 0.20 |
| RANTES | 6891.99 (14598.71±2442.89) | 108.16 | 0.02 |
| TNF-α | 34.36 (96.85±43.29) | 50.53 | 1.47 |
| VEGF | 6.92 (128.05±58.77) | 207.53 | 30.0 |
| These were sampled on the fifth day. Serum reference values indicate a mean±a standard deviation in five healthy subjects in our institution as a control. FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IP, interferon-γ-inducible protein; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; OOR, out of range; PDGF, platelet-derived growth factor; RANTES, regulated on activation, normal T-cell expressed and secreted; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor. |
This report presents the results of cytokine profile analysis in a patient with cardiac tamponade and severe LV dysfunction following COVID-19. The results were consistent with cytokine storm originating from the intrapericardial cavity. To the best of our knowledge, this is the first report to describe the cytokine profile of pericardial effusion in a pediatric patient with COVID-19.
COVD-19 can lead to an excessive immune response, which may be described as MIS-C in children, a condition similar to Kawasaki disease.2) This case met the definition of MIS-C,4, 5) but its timing and symptoms were not typical. The symptoms and signs could also be diagnosed as myocarditis associated with COVID-19. Even in the setting of myocarditis, however, direct damage to the myocardial cells is not always the solitary mechanism of the disease.6) Other possible mechanisms have been nominated, such as damage to vascular endothelial cells and immune system hyperactivity, and previous reports regarding the myocardial tissue of COVID-19-associated LV dysfunction suggested that etiology of the disorder is more related to immunological hyperactivity than direct damage to the myocardium.6) Therefore, the underlying mechanism appears to be closely associated with excessive activation of the immune system, regardless of classification of the clinical condition in this patient.
Only a limited number of cytokine types increased their serum levels in this patient, including IL-1ra, IL-8, IL-12, and interferon (IFN)-γ. In the previous report of cytokine analysis in fulminant myocarditis, elevated levels were documented in IL-10, IL-17, IL-12, vascular endothelial growth factor (VEGF), IL-4, IFN-γ, and IL-13.7) The fact that characteristics in serum analysis in our patient were dissimilar to those in fulminant myocarditis may indicate different pathogenesis of illness. Another possibility is that serum cytokines were in the process of elevation being transferred from the intrapericardial space. The elevated serum levels of IL-12 and IFN-γ may support this assumption, while it is unknown why the serum levels of many others were within their normal ranges.
Most cytokines in our patient showed a marked increase in concentration in the pericardial fluid compared with the serum, suggestive of cytokine storm originating from the intrapericardial region. The IL-6 level was the most prominent parameter; its value in the pericardium being 382 times higher than that in the serum. A previous report on pericardial fluid in viral pericarditis showed a limited increase in IL-6 in pericardial effusion8); therefore, we consider that the disproportionally high IL-6 concentration in our case was atypical for simple viral pericarditis.
IL-6 plays an important role in the excessive immunoreaction to SARS-CoV-2. Reportedly, signaling from the angiotensin II and angiotensin I receptors induced by SARS-CoV-2 activates the IL-6 amplifier, which is a possible mechanism underlying cytokine storm through the activation of nuclear factor kappa B and transcription factor 3.3, 9) Interestingly, a case report of an adult with cardiac tamponade caused by COVID-19 showed a similar cytokine profile in which the IL-6 concentration in the pericardial effusion was disproportionately high compared to that in the serum.10) In the same report, the authors concluded that the COVID-19-related cytokine storm was limited to the pericardium. In line with this, we suggest that immune system hyperactivity following COVID-19 in children can also originate from and be exclusively localized in the intrapericardial area. Another study in patients with idiopathic pericarditis described markedly increased IL-6 levels in the pericardial effusion.11) Although the cause of pericarditis in those patients was unclear, they may share immunological processes similar to our own patient.
We also detected great differences between serum and intrapericardial levels of IFN-γ-induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1, VEGF, and eotaxin in our patient. Some previous studies reported elevated serum concentration of these cytokines and chemokines in severe COVID-19,12–14) whereas such increases were limited in our patient. IP-10, MCP-1, and eotaxin do activate lymphocytes, monocytes, and eosinophils, but no cellular components were found in the pericardial fluid of our patient. This may reflect that the large volume of pericardial fluid made it difficult to detect them.
The present case appears to demonstrate an excessive immunoreaction after COVID-19, but its pattern does not seem to be common in hyperactive immune responses following COVID-19, especially for MIS-C. The symptoms and complications of MIS-C are not always identical among patients15); still, they can be similar to those of Kawasaki disease and can appear systemically throughout the body. In such occasions, the serum cytokine profile can resemble Kawasaki disease, even with some differences.1) Obtaining multiple samples from bodily fluid would be unrealistic. Often, where and how cytokines are localized is not evident from systemic symptoms and signs. Further study is therefore warranted to clarify the variety of excessive immune responses following COVID-19 in children.