Chylothorax is a well-known and serious postoperative complication in children undergoing congenital cardiac surgery, occurring with an incidence between 0.6 and 6.6%.1, 2) Possible causes of chylothorax are injury to the thoracic lymph duct, increasing central venous pressure, congenital lymphatic malformation, and chromosomal abnormality.1, 2) In some patients with congenital heart disease, chylothorax happens even during the preoperative period due to increased central venous pressure. Chylothorax increases both mortality and morbidity. In particular, it can cause prolonged intensive care unit (ICU) stay and need for mechanical ventilation. Those patients are susceptible to infection, venous thrombosis, and malnutrition.2, 3) There is no overall consensus regarding the optimal treatment of chylothorax, while fat-free or low-fat diet, nil per os (NPO) with total parenteral nutrition (TPN), administration of steroids or octreotide, pleurodesis, and surgical procedures are generally adopted and widely used as current treatments.1, 2, 4, 5)
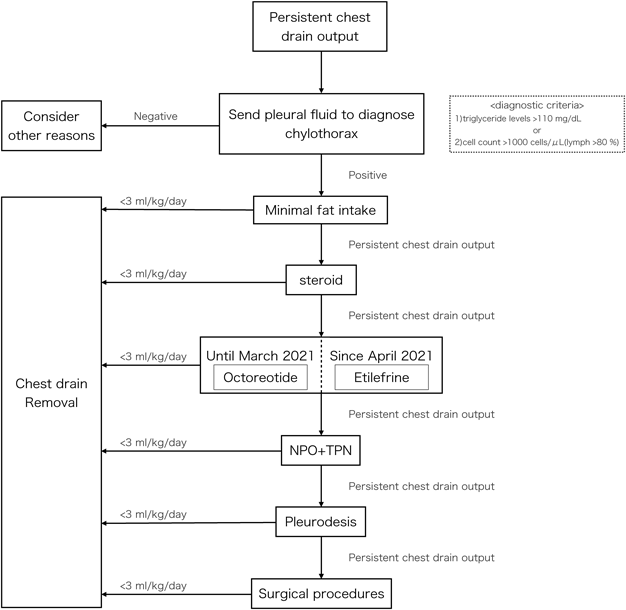
Although our institution has not set a strict protocol for chylothorax treatment, the practical approach adopted by us is summarized in Fig. 1. We initially attempted a low-fat diet once diagnosing the patient as chylothorax, and prednisolone was administered in those not responding to a low-fat diet. Following this, octreotide was administered as the next step. NPOs with TPN were usually introduced when patients had not responded to other medical therapies. Pleurodesis was considered necessary when dietary adjustments and medical therapy were ineffective. Further intervention, such as ligation of the lymph duct or lymphangiography, was considered when chylothorax did not improve after the conservative treatments. The threshold for drain removal was determined to be less than 10 mL/day (3–5 mL/kg/day) in neonates and <3 mL/kg/day in older children. When the amount of pleural effusion did not come down below the criterion for drain removal, we discussed additional treatments still further.
Octreotide is a somatostatin analog that is administered frequently off-label for several conditions, including chylothorax. Hypotension and necrotizing enterocolitis are known side effects,6) and this drug should be used with caution, especially in neonates. Octreotide was previously regarded as the next step at our institution when pleural effusion did not resolve after initiation of dietary adjustments and administration of steroids. Though this drug is currently widely used for chylothorax, we have experienced some patients in whom octreotide was less than effective. In fact, some of those did not tolerate continued administration of octreotide due to hypotension as its side effect.
It has been reported that etilefrine is effective for chylothorax after esophageal surgery in children and adults,2, 7) and for idiopathic chylothorax.8) Etilefrine infusion is also an off-label use for chylothorax, and it has side effects such as hypertension, tachycardia, and arrhythmia.2, 7–9)
On the other hand, there have been no reports of etilefrine infusion in patients with congenital heart disease. This report describes four patients with refractory chylothorax who underwent congenital cardiac surgery at our institution between April 2021 and March 2022; safety and efficacy of etilefrine were evaluated in these patients. Another two patients with congenital heart disease received etilefrine infusion during this period, but either of them was excluded because drainage output through the chest tube could not be evaluated precisely due to intrathoracic lavage being performed for open chest management after resuscitation. In this report, chylothorax was defined as refractory when not responding to treatments by dietary adjustments and use of steroids and, at the same time, when deemed to require additional treatment. We administered etilefrine, instead of octreotide, in our four patients.
All patients’ information are provided in Table 1.
Table 1 Patients characteristics and clinical courses| Case | Sex | Birth weight (gram) | Cardiac diagnosis | Underlying disease | Operation | Age at surgery (days old) | Duration of drain placemant (days) | Duration of etilefrine administration (days) |
|---|
| 1 | Female | 2097 | Supracardiac TAPVC | None | TAPVC repair | 12 | 43 | 15 |
| 2 | Male | 2892 | TGA with intact ventricular septum | None | arterial switch (Lecompte) | 6 | 24 | 14 |
| 3 | Male | 2660 | incomplete AVSD, common atrium, IVC interruption | Left isomerism | mPAB+PDA ligation | 12 | 12 | 33 |
| 4 | Male | 2014 | complete AVSD | 21 trisomy | mPAB+PDA ligation | 14 | 37 | 33 |
| AVSD, atrioventricular septal defect; IVC, inferior vena cava; mPAB, main pulmonary arterial banding; PDA, patent ductus arteriosus; TAPVC, total anomalous pulmonary venous connection; TGA, transposition of the great arteries |
Case 1 (Fig. 2a)
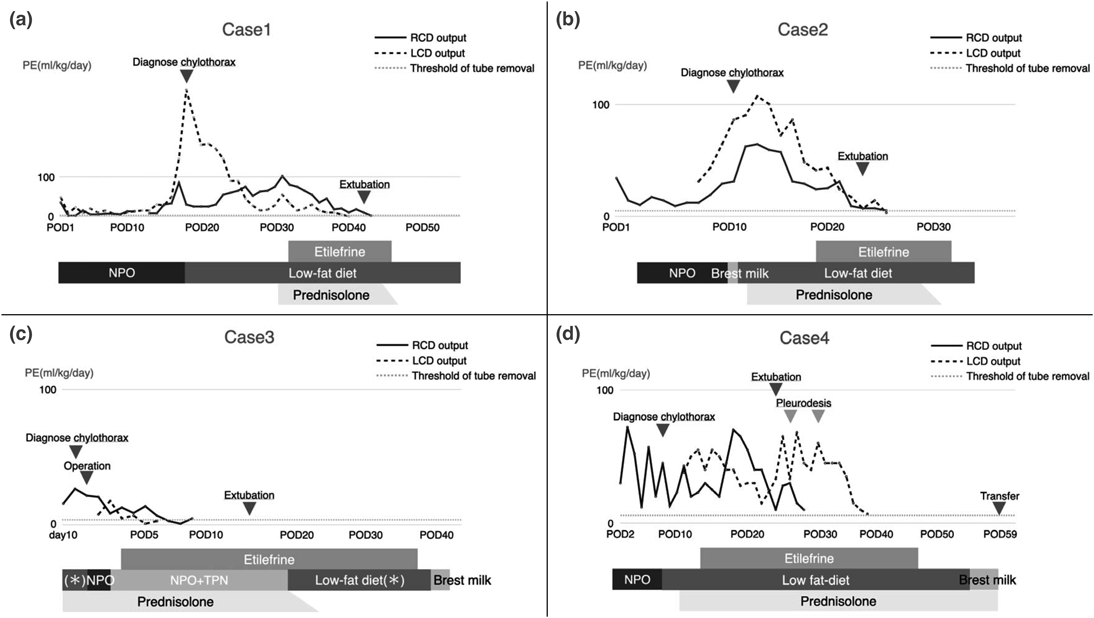
A female child, born at 37 5/7 weeks gestational age (GA), delivered by cesarean section. She was transferred from another hospital on the second day of life (DOL) with a postnatal diagnosis of supracardiac total anomalous pulmonary venous connection (TAPVC). The patient underwent TAPVC repair on DOL 12. Following surgery, she was hemodynamically unstable, and a large pleural effusion was noted, which required a continuous indwelling chest drain. She was diagnosed as having chylothorax by fluid analysis, which revealed an elevated lymphocyte fraction (96%) on the eighteenth postoperative day (POD). Enteral feeding was initiated with low-fat milk on the same day. Prednisolone was subsequently started at 2 mg/kg/day for treating chylothorax. Despite these treatments, right- and left-sided pleural effusion remained around 80 and 35 mL/kg/day, respectively. On POD 31, etilefrine infusion was initiated at 0.7 µg/kg/h and titrated by 0.15 µg/kg/h every 24 h up to 1.0 µg/kg/h, while circulatory parameters were continuously monitored in the ICU to detect side effects, if any, such as hypertension, tachycardia, and arrhythmia. There were no such signs of side effects, and right- and left-sided pleural effusion decreased over 10 days and 2 weeks, respectively. The patient was extubated on POD 42. The right- and left-sided chest tubes were removed on POD 40 and 43, respectively. Etilefrine infusion was weaned off on POD 46.
Case 2 (Fig. 2b)
A male child, born at 37 4/7 weeks GA via vaginal delivery at a previous hospital. Shortly after birth, the patient developed cyanosis and was intubated because of severe hypoxemia. His heart disease was postnatally diagnosed as transposition of the great arteries (TGA) with an intact ventricular septum. The patient was then transferred to our hospital for surgery. On DOL 6, the patient underwent the arterial switch operation. A large pleural effusion accumulated on the left side of the chest, requiring insertion of a chest tube into the left-sided pleural space on POD 8. Breast milk feeding was initiated on POD 10. The following day, the appearance of output through the chest tube changed to a milky one. Although a lymphocyte fraction measured was 57%, we diagnosed his condition as chylothorax based on elevated triglyceride levels (140 mg/dL) on the fluid analysis. A low-fat diet was initiated on the same day, and prednisolone (2 mg/kg/day) was started on POD 12. However, the right- and left-sided pleural effusion remained at approximately 30 and 45 mL/kg/day, respectively. Hence, etilefrine infusion was initiated at 0.7 µg/kg/h on POD 19 and titrated by 0.3 µg/kg/h every 24 h up to 1.0 µg/kg/h, on continuous monitoring to detect side effects in the ICU. The pleural effusion declined over 6 days. The patient was extubated on POD 22, and the chest tubes were removed on POD 24. Etilefrine was discontinued on POD 33. No side effects were noted during infusion of the agent.
Case 3 (Fig. 2c)
A male child, born at 37 weeks GA via vaginal delivery, had prenatal diagnosis of left atrial isomerism, incomplete atrioventricular septal defect (AVSD), common atrium, and inferior vena cava interruption. The patient developed respiratory failure after delivery and required intubation in a neonatal ICU (DOL 0). Breast milk feeding was initiated on DOL 3. Subsequently, pleural effusion accumulated on both sides of the chest, and breast milk feeding was discontinued on DOL 6. The following day, prednisolone was administered at a dose of 2 mg/kg/day. On DOL 10, although low-fat milk had been introduced, the patient respiratory complications worsened due to a right-sided pleural effusion, requiring insertion of a chest tube into the right pleural space. Fluid analysis revealed chylothorax, as evidenced by a white blood cell (WBC) count of 3983 cells/µL (97% lymphocytes). On DOL 12, main pulmonary arterial banding (mPAB) and ligation of patent ductus arteriosus (PDA) were performed. A large pleural effusion accumulated on the left side of the chest, requiring insertion of another chest tube into the left pleural space on POD 1. Subsequently, the right- and left-sided pleural effusion remained around 10 and 5 mL/kg/day, respectively. The chest tubes could not be removed during the early postoperative period. Thus, etilefrine infusion was initiated at 0.3 µg/kg/h on POD 4 and titrated by 0.35 µg/kg/h approximately every 12 h up to 1.0 µg/kg/h, on continuous monitoring to detect side effects in the ICU. Left- and right-sided pleural effusion ceased over 3 days and 6 days, respectively. Hence, the left- and right-sided chest tubes were removed on POD 6 and 9, respectively. The patient was extubated on POD 13. Etilefrine was weaned off on POD 37 without any side effects.
Case 4 (Fig. 2d)
A male child, born at 37 5/7 weeks GA by cesarean section. The patient was postnatally diagnosed with complete AVSD in the setting of trisomy 21. On DOL 14, the patient underwent mPAB and PDA ligation. On POD 2, a right-sided chest tube was inserted because of pleural effusion. On POD 8, he was diagnosed with chylothorax following a fluid analysis revealing a WBC of 1380 cells/µL (94% lymphocytes). Hence, enteral feeding was initiated with low-fat milk. Subsequently, a left-sided chest tube was also inserted because of accumulation of pleural effusion, and prednisolone (2 mg/kg/day) was initiated on POD 11. The right- and left-sided pleural effusion remained at over 30 and 50 mL/kg/day, respectively. Etilefrine infusion was initiated at 0.5 µg/kg/h on POD 14 and titrated by 0.5 µg/kg/h approximately every 24 h up to 1.0 µg/kg/h, on continuous monitoring to detect side effects. The patient was extubated on POD 24. The pleural effusion from the right-sided pleural effusion decreased over 10 days and was removed on POD 28. In contrast, the amount of drainage from the left-sided pleural cavity remained high at over 45 mL/kg/day. Therefore, pleurodesis was performed on the left side of the chest on PODs 26 and 30. Subsequently, pleural effusion decreased over approximately 2 weeks, and the left-sided tube was removed on POD 37. Etilefrine was weaned off on POD 47 without side effects. He was transferred back to the previous hospital on POD 59.
Chylothorax is one of the common causes of pleural effusion, and may result in significant losses of bodily fluids, lymphocytes, and proteins. Chylothorax is responsible for high in-hospital mortality (odds ratio [OR], 2.13; 95% confidence interval [CI], 1.75–2.61),10) and prolongs the length of mechanical ventilation and ICU stay. Additionally, it is associated with a higher rate of reintubation; the event increases challenging immunological issues such as sepsis and malnutrition. Chylothorax is known to occur more frequently in the younger age groups, particularly in neonates (OR, 7.03; 95% CI, 4.61–10.7).10) Moreover, some particular surgeries have higher incidences of postoperative chylothorax: the Fontan type operation (5.7%), the arterial switch operation for TGA (4.3%), repair of congenital aortic arch anomalies (3.7%), and repair of TAPVC (3.7%).10)
Precise diagnosis is the key to early treatment. There are currently no established criteria for chylothorax, but it is generally diagnosed based on the following features of pleural effusion described in previous reports: 1) triglyceride levels >110 mg/dL, 2) absolute cell count >1000 cells/µL, and 3) lymphocytes >80%.4, 11) In neonates, however, diagnosing chylothorax remains challenging since definition of chyle triglyceride level is unclear at this stage of life.12) In addition, some medical reports state that the diagnosis of chylothorax requires minimum fat intake.4, 11)
The current treatment strategy includes NPO, fat-free or low-fat diet, NPO with TPN, medical therapy, pleurodesis, and surgical treatment, such as thoracic duct ligation.4, 5) Generally, noninvasive treatment precedes surgical treatment. Steroid therapy may cause infection, hyperglycemia, hypertension, and delayed wound healing as side effects. As mentioned in the introduction, we had no pre-defined treatment protocol for chylothorax, and the timing of additional treatment varied from one case to another. In this series, we initiated use of etilefrine following conventional therapy. Pleurodesis could have been the next step after addition of etilefrine remedy. Surgical procedures to significant hemodynamic impediments should also have been considered as far as such residual lesions were causing increased venous pressure.
Etilefrine is a sympathomimetic drug used to treat postural hypotension and stimulates α and β adrenergic receptors. It causes systemic smooth muscle contraction and decreases chyle flow by constricting the thoracic ducts.7, 8) We infused etilefrine via a central venous catheter, initially at 0.3–0.7 µg/kg/h, and increased the dose by 0.15–0.5 µg/kg/h approximately every 12–24 h up to 1.0 µg/kg/h with careful monitoring in the ICU based on previous reports.2, 9) There was no established regimen for its dose; the plan for initiation, titration, and reduction was carefully determined in each case. Moreover, the use of etilefrine is also still off-label, and therefore we obtained approval from the ethics committee of our institution and obtained consent from the patient's guardian.
To the best of our knowledge, this is the first report to investigate efficacy and safety of etilefrine for chylothorax in patients with congenital heart disease. In our study, etilefrine plus conventional therapy seemed to be effective in Cases 1, 2, and 3. When etilefrine was initiated, their pleural effusion was not below the threshold for removal. The pleural effusion decreased over 5–14 days after the addition of etilefrine. In Case 4, in contrast, adding etilefrine to conventional therapy was insufficient, and the left-sided pleural effusion persisted over 45 mL/kg/day. According to a previous report, trisomy 21 can sometimes have thoracic duct malformation,13) which might influence the large amount of pleural effusion in our case. There are some medical reports on the successful management of chylothorax with a combination therapy of octreotide, pleurodesis, and etilefrine.9, 14) We added the pleurodesis as the subsequent step in Case 4. After pleurodesis, the pleural effusion on the left side resolved.
We recommend continuous monitoring during use of etilefrine, though side effects of this drug are relatively rare, such as hypertension (<5%), tachycardia, and arrhythmia. In our patients, there were no such episodes when etilefrine was used concomitantly with inotropes. As mentioned earlier, octreotide has demonstrated some evidence of efficacy in the management of chylothorax. That is why octreotide is widely used for chylothorax. It is noteworthy, however, that a previous report described octreotide caused hypotension (12%) and necrotizing enterocolitis (2%) in neonates.6) In particular, hypotension is a concern in patients hemodynamically unstable and on inotropic drugs; they require careful administration. Moreover, the elimination half-life of octreotide is approximately 90–120 min.15) For these reasons, we think octreotide would be unsuitable for patients with hemodynamic instability. Therefore, octreotide should be carefully administered especially in neonates, as well as in those requiring infusion of inotropes, undergoing surgery for congenital heart disease. When considering the effect on blood pressure, hypertension may pose a less severe effect on the outcome than hypotension does in patients with compromised hemodynamics. Thus, it is possible that etilefrine is safer than octreotide when treating chylothorax. Furthermore, etilefrine is significantly more cost-effective compared to octreotide. As far as etilefrine proves to be effective, it can potentially reduce healthcare costs. If hemodynamic instability is the main concern, it would be a good idea to start with etilefrine. In case one drug regimen turned out to be less effective, a combination therapy might be implemented which can be advantageous as indicated by a previous report.8)
Our report has some limitations because of the small number of cases. Although the pleural effusion was above the removal threshold when etilefrine was initiated, there was a slight downward trend of the effusion at that time. Additionally, it is difficult to propose a new treatment protocol for chylothorax based on this report.
Our cases demonstrated that the administration of etilefrine was safe and appeared effective for chylothorax. Therefore, etilefrine might be an alternative nonsurgical treatment for chylothorax. Further research is needed to determine which treatment option is more effective for chylothorax in patients with congenital heart disease.
謝辞Acknowledgments
We thank Edward Neil for his assistance with editing the manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Ethical Declarations
This case series and the use of etilefrine was approved by the Ethics Committee of the Sakakibara Heart Institute. The patients and their guardians were provided the opportunity to opt out of this study, and the identity of the patients was protected. Etilefrine has not been approved for chylothorax, and written consent to use of etilefrine was obtained from the patient’s guardians in each case prior to initiation of etilefrine infusion.
Author Contribution
Hiroaki Sammori drafted and prepared the initial manuscript and made the corresponding revisions. Kazuhiro Shoya revised the manuscript and made scientific contributions. Yu Matsumura reviewed the manuscript and made scientific contributions. Naoki Wada and Tadahiro Yoshikawa reviewed the manuscript. All authors have approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
引用文献References
1) Milonakis M, Chatzis AC, Giannopoulos NM, et al: Etiology and management of chylothorax following pediatric heart surgery. J Card Surg 2009; 24: 369–373
2) Muniz G, Hidalgo-Campos J, Valdivia-Tapia MDC, et al: Successful management of chylothorax with etilefrine: Case report in 2 pediatric patients. Pediatrics 2018; 141: e20163309
3) Ismail SR, Kabbani MS, Najm HK, et al: Impact of chylothorax on the early post operative outcome after pediatric cardiovascular surgery. J Saudi Heart Assoc 2014; 26: 87–92
4) Soto-Martinez M, Massie J: Chylothorax: Diagnosis and management in children. Paediatr Respir Rev 2009; 10: 199–207
5) Saad D, Makarem A, Fakhri G, et al: The use of steroids in treating chylothorax following cardiac surgery in children: A unique perspective. Cardiol Young 2022; 1: 1–6
6) Testoni D, Hornik CP, Neely ML, et al: Best Pharmaceuticals for Children Act—Pediatric Trials Network Administrative Core Committee: Safety of octreotide in hospitalized infants. Early Hum Dev 2015; 91: 387–392
7) Guillem P, Billeret V, Houcke ML, et al: Successful management of post-esophagectomy chylothorax/chyloperitoneum by etilefrine. Dis Esophagus 1999; 12: 155–156
8) Kinoshita S, Shoya K, Shimotakahara A, et al: Etilefrine infusion for idiopathic chyle leakage in a critically ill child. Pediatr Int 2022; 64: e14850
9) Tomobe Y, Mizuguchi U, Shimotakahara A, et al: Combination therapy with etilefrine and pleurodesis for refractory congenital chylothorax. Biomed Hub 2020; 5: 907–911
10) Mery CM, Moffett BS, Khan MS, et al: Incidence and treatment of chylothorax after cardiac surgery in children: Analysis of a large multi-institution database. J Thorac Cardiovasc Surg 2014; 147: 678–686.e1
11) Büttiker V, Fanconi S, Burger R: Chylothorax in children: Guidelines for diagnosis and management. Chest 1999; 116: 682–687
12) Senarathne UD, Rodrigo R, Dayanath BKTP: Milky pleural effusion in a neonate and approach to investigating chylothorax. BMJ Case Rep 2021; 14: e245576
13) Buchwald MA, Laasner U, Balmer C, et al: Comparison of postoperative chylothorax in infants and children with trisomy 21 and without dysmorphic syndrome: Is there a difference in clinical outcome? J Pediatr Surg 2019; 54: 1298–1302
14) Ohkura Y, Ueno M, Iizuka T, et al: New combined medical treatment with etilefrine and octreotide for chylothorax after esophagectomy: A case report and review of the literature. Medicine (Baltimore) 2015; 94: e2214
15) Basma H, Saras S, Khalid H: Somatostatin analogues for the treatment of hyperinsulinaemic hypoglycemia. Ther Adv Endocrinol Metab 2020; 11: 2042018820965068