ECG Screening for School-Aged Children
Similar to Japan, Taiwan has been conducting a long-term cardiac screening program for school-aged children with the support of the Cardiac Children Foundation, Taiwan. The main method used for cardiac screening in school-aged children was electrocardiography (ECG), which has shown significant effectiveness. Numerous studies have been published in this field, including research on Cardiac conduction disturbance detected in a pediatric population, long-term outcomes of pediatric sinus bradycardia, and cardiac screening for high risk sudden cardiac death in school-aged children.1–3)
COVID-19 Pandemics and Vaccination
The COVID-19 pandemic has had a tremendous impact on human life worldwide over the past three years. This global outbreak has not only caused a large number of infections and deaths but has also fundamentally changed human lifestyles. Over the past two years, Taiwan’s government has effectively controlled the infection rate, resulting in relatively fewer cases of infection.4) However, with the emergence and spread of the Omicron variant, the number of confirmed cases has surged, and it was estimated that more than one-third of the population in Taiwan has been infected with COVID-19 by 2022.4) Similar to other countries, we also relied on vaccines as the primary tool to prevent severe cases of COVID-19.5) However, heart-related side effects have been observed from vaccines after large-scale vaccination campaigns. In June 2021, the U.S. Food and Drug Administration (FDA) issued a warning indicating that mRNA vaccines may cause myocarditis, particularly more commonly observed in young individuals.6, 7) The U.S. CDC’s Adverse Event Reporting System compiled cases possibly related to vaccine-induced myocarditis.5) It was found that the prevalence of myocarditis was highest in males after receiving the second dose of the vaccine, particularly in young individuals aged 12 to 24 years, with an estimated occurrence of 52 to 105 cases per million people.8) In the nationwide prevalence study conducted in Israel, it was similarly observed that males between the ages of 16 and 24 had the highest incidence of myocarditis, with an estimated rate of 15 cases per 100,000 people. When taking background rates into account, the myocarditis incidence could potentially increase by 5.3 times.9) Furthermore, regarding the timing of occurrences, they also found that the highest incidence of myocarditis was observed within two to seven days after receiving the second dose of the vaccine.10)
Background of Screening for Post-Vaccine Cardiac Adverse Effects
Due to the negative news surrounding these vaccines, there was significant resistance among the public, students, and parents towards receiving vaccinations, especially in the context of the government’s preparations for large-scale administration of the BNT162b2 vaccine on the younger population. Therefore, we endeavored to explore potential measures for early detection of myocarditis cases. However, when we proposed the plan of implementing ECG screening to detect cardiovascular adverse effects in school-aged children, we encountered numerous doubts and inquiries. Let us now examine the key questions to determine the feasibility and value of conducting such a screening program.
Doctor Kaltman have defined the key questions for determining whether it is worthwhile to conduct a screening program.11) First, it should be an important issue and, we need to identify the high-risk population and focus on screening these individuals. Secondly, we must have effective screening tools that are accurate, easy to use, non-invasive, affordable, and capable of early detection. Thirdly, the screened disease should be treatable, and early intervention should lead to improved survival rates.
In addressing the first aspect, BNT162b2-related myocarditis represents a significant public health concern that can impact vaccination rates. The high-risk group has been pinpointed as the young male population following the administration of the second vaccine dose. Regarding the third aspect, timely identification and early treatment of myocarditis can reduce morbidity and mortality. Turning to the second aspect, the disease typically manifests two days after receiving the vaccine, affording us a crucial window for screening. Consequently, there is a justified need for screening for post-vaccine myocarditis. However, determining the optimal and cost-effective screening tool remains to be elucidated.
Screening Tools of Post-Vaccine Cardiac Adverse Effects
Firstly, we need to understand how myocarditis is diagnosed. In 2021, the U.S. Centers for Disease Control and Prevention (CDC) redefined the diagnostic criteria for myocarditis cases.6) For potential cases, one symptom (chest pain, shortness of breath, palpitations, and fainting) must be present along with one laboratory finding (elevated cardiac enzymes, abnormal electrocardiogram, impaired cardiac function, and magnetic resonance imaging consistent with myocarditis). For confirmed cases, one symptom and either histopathological confirmation or elevated cardiac enzymes with magnetic resonance imaging diagnosis are required. As magnetic resonance imaging and echocardiography is relatively expensive, more favorable screening tools may include symptom screening, blood tests for cardiac enzymes, or electrocardiogram screening.
At that time in Taiwan, the government provided each vaccine recipient with an informational leaflet, highlighting symptoms to watch out for. However, a significant proportion of individuals experienced cardiac-related symptoms after vaccination. Therefore, whether other screening methods, such as electrocardiogram screening, can be used to detect cardiac-related complications in school-aged adolescents early after vaccination, is a focal point of our research.
Screening Method for Post-Vaccine Cardiac Adverse Effects
In Taiwan, there is a specific process for administering the COVID-19 BNT162b2 vaccine to school-aged children. All high school students in the same school received the BNT162b2 vaccine on the same day within the school premises. Additionally, the vaccination schedule was tightly packed, with all high school students across the country receiving their first dose of the vaccine within two weeks, followed by the second dose after 12 weeks, completed within another two weeks.
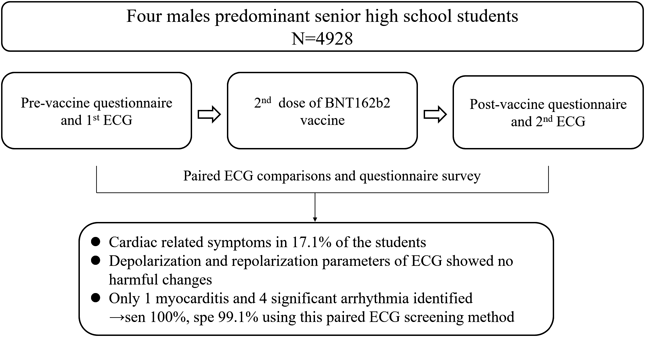
Therefore, our research design involved conducting the first questionnaire survey and performing pre-vaccination electrocardiogram (ECG) examinations before administering the 2nd dose of the vaccine.12) After the administration of the second dose of the BNT162b2 vaccine, we conducted the second questionnaire survey and perform ECG examinations once again, followed by a comparison of the ECG readings before and after vaccination.
Fig. 1 is an on-site photo where we have set up the school’s sports center with 50 partitions, each equipped with an operator and an electrocardiogram (ECG) machine. Consequently, we could conduct ECG examinations for 50 students simultaneously. We used PCA 500 12-lead ECG recorder (QT Medical Inc.). ECG data were transferred wirelessly to the cloud (QT Cloud; QT Medical Inc.) and analyzed using an ECG diagnostic system (QTM-DX; QT Medical, Inc.). The ECG data was first checked by QTM-DX analyzer and doubly checked by pediatric cardiologists on the web. The pre- and post-vaccine ECGs were compared using the serial comparison function of QTM-DX. Table 1 shows the criteria of the significant ECG change.
Table 1 The criteria of significant ECG change set for serial comparison of pre- and post-vaccine ECGs by QTM-DX| Premature ventricular beats (PVC) |
| New PVC detected |
| Number of PVCs increase more than 2 |
| PVC couplets, triplets or runs |
| More than 2 PVC morphologies |
| Premature atrial beats (PAC) |
| New PAC detected |
| Number of PACs increase more than 2 |
| PAC couplets, triplets or runs |
| Heart rate change |
| Heart rate increase >20 beats per minute (bpm) and current heart rate >100 bpm |
| Heart rate decrease >20 bpm and current heart rate <60 bpm |
| Extreme sinus bradycardia |
| Heart rate below 40bpm |
| Atrial arrhythmia |
| New atrial fibrillation, flutter, or tachycardia detected |
| Atrioventricular block |
| PR interval increase >30 ms |
| 2nd or complete atrioventricular block |
| Bundle branch block or intraventricular conduction delay |
| New right bundle branch block detected |
| New left bundle branch block detected |
| QRS duration increase >15 ms and QRS duration >110 ms |
| Q wave change |
| Q wave duration≥20 ms at lead V2, V3 |
| Q wave duration≥30 ms and Q wave amplitude >0.1 mV at other leads |
| QS complex in contiguous leads |
| QRS change |
| QRS axis change by >45 degrees |
| Sum of limb leads’ (R+S) decrease by >30% |
| Sum of chest leads’ (R+S) decreased by >30% |
| ST change |
| ST elevated ≥0.2 mV compared to the baseline |
| ST depressed ≥0.2 mV compared to the baseline |
| T wave change |
| New T wave inversion detected |
| T wave amplitude >10 mm |
| Prolonged QT |
| QTc interval increase >20 ms |
| QTc interval >470 ms |
| Courtesy provided by Chiu SN, et al. Eur J Pediatr 2023: 1–8.12) |
Results of ECG Screening for Post-Vaccine Cardiac Adverse Effects
Among 7934 eligible students, 4928 (62.1%) completed both pre- and post-vaccine ECGs (Fig. 2). The male-to-female ratio was 13 : 1, and the mean age was 16.7±0.9 years. Underlying medical conditions were present in 109 (2.2%) of the participants, with simple congenital heart disease, mitral valve prolapse, and arrhythmia being the most common.
From the questionnaire, the incidence of cardiac-related symptoms was 5.7% after the 1st dose and 17.1% after the second dose of the BNT162b2 vaccine, with palpitations and chest pain being the most common symptoms. When comparing ECG parameters, there was a significant increase in heart rate after the vaccine, with a mean increase of 2.6 beats per minute (bpm). Additionally, the QRS duration, QT, QTc, and QTcf intervals all decreased significantly after the vaccine. Based on serial comparisons of pre- and post-vaccine ECGs, 51 (1.03%) students were identified as having significant ECG changes after vaccination. ST-T changes were the most common, present in 72.5% of the students, followed by arrhythmia (13.7%), bundle branch block (5.9%), and abnormal QRS (3.9%) and QT intervals (3.9%). Hospital transfer was arranged for these patients, and 5 of them were deemed to have significant adverse events. One student had subclinical myocarditis with premature ventricular beats, and although his troponin T level increased to 19 ng/L, it spontaneously recovered without admission. The other 4 students had arrhythmia, with 2 experiencing sinus bradycardia, 1 with atrial tachycardia, and 1 with premature ventricular beats.12) Among the remaining 4877 students with negative screening results, detailed information on post-vaccine cardiac adverse effects was collected from school nurses and the school vaccination reporting system of the Health Bureau of Taipei City government 1 month after vaccination. All these students were confirmed to have no adverse cardiac events requiring hospital admission.
Accuracy and Cost Analysis
We also conducted sensitivity and specificity analysis and compared different screening methods, including serial comparison as used in this study, post-vaccine ECG screening alone, and post-vaccine ECG combined with any cardiac symptoms. The serial comparison method showed a sensitivity of 100%, specificity of 99.1%, positive predictive value 9.8% and negative predictive value 100%. When using post-vaccine ECG screening alone, the specificity dropped to 86%, resulting in a low positive predictive value of 0.72%. The addition of cardiac symptoms in the post-vaccine ECG increased the specificity to 97.5%, but the sensitivity dropped to 20% and positive predictive value remained low 0.79%. Therefore, the serial comparison method is the most appropriate screening approach for detecting post-vaccine cardiac adverse events.12) We then performed a cost and benefit analysis, revealing that the screening cost was US$14.3 per student, meaning the cost to identify a case of cardiovascular adverse events was US$14,094. However, as indicated in the current study and previous reports, most adverse events were mild. Utilizing data from the Israel nationwide survey study, detecting a case of severe myocarditis would incur an expense of US$430,462.12)
Apart from electrocardiogram (ECG) screening, are there any other screening methods available? In one Israeli hospital, a before-and-after comparison trial was conducted on healthcare workers receiving the fourth dose of the COVID-19 BNT162b2 mRNA vaccine.13) Blood samples were taken to test for Troponin T levels before and after vaccination. Among the 324 participants, two individuals showed a slight increase in Troponin T levels, but both values were <25 ng/L. Furthermore, subsequent ultrasound, ECG, and magnetic resonance imaging examinations for these two participants were all normal. This finding further demonstrates that most myocardial injuries caused by the BNT162b2 vaccine are relatively mild.
Although cardiac-related symptoms were common, the incidence of myocarditis, even subclinical cases, was very low. The ECG parameters of depolarization and repolarization exhibited no significant abnormal changes, and arrhythmias were rare after vaccination in this healthy young population. ECG screening with pre- and post-vaccine comparison demonstrated high sensitivity and specificity. Nevertheless, cost and benefit considerations needed to be justified. However, this ECG screening method can serve as a reference for future medical events that may require screening.
Conflicts of Interest
None.
Author Contribution
Shuenn-Nan Chiu conceptualized the study, collected the data, analyzed the initial study, drafted and reviewed and revised and final proved the manuscript Yih-Sharng Chen, Yu-Chuan Hua, and Jou-Kou Wang: conceptualized and designed the study, coordinated and collected the data, analyzed the initial study, and reviewed and revised and final proved the manuscript.
引用文献References
1) Chiu SN, Wang JK, Wu MH, et al: Taipei Pediatric Cardiology Working Group: Cardiac conduction disturbance detected in a pediatric population. J Pediatr 2008; 152: 85–89
2) Chiu SN, Lin LY, Wang JK, et al: Long-term outcomes of pediatric sinus bradycardia. J Pediatr 2013; 163: 885–889.e1
3) Liu HW, Huang LW, Chiu SN, et al: Cardiac screening for high risk sudden cardiac death in school-aged children. Acta Cardiol Sin 2020; 36: 641–648
4) Taiwan Centers for Disease Control: Prevention and Control of COVID-19 in Taiwan. 2021. https://www.cdc.gov.tw/En/Category/Page/0vq8rsAob_9HCi5GQ5jH1Q
5) Gargano JW, Wallace M, Hadler SC, et al: Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 977–982
6) Centers for Disease Control and Prevention (CDC): Advisory Committee on Immunization Practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines. 2021. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-2006.html
7) Marill MC: FDA to add myocarditis warning to mRNA COVID-19 vaccines. Medscape, 2021. https://www.medscape.com/viewarticle/953647
8) Oster ME, Shay DK, Su JR, et al: Myocarditis cases reported after mRNA-based COVID-19 Vaccination in the US from December 2020 to August 2021. JAMA 2022; 327: 331–340
9) Mevorach D, Anis E, Cedar N, et al: Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med 2021; 385: 2140–2149
10) Witberg G, Barda N, Hoss S, et al: Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med 2021; 385: 2132–2139
11) Kaltman JR, Thompson PD, Lantos J, et al: Screening for sudden cardiac death in the young: Report from a national heart, lung, and blood institute working group. Circulation 2011; 123: 1911–1918
12) Chiu SN, Chen YS, Hsu CC, et al: Changes of ECG parameters after BNT162b2 vaccine in the senior high school students. Eur J Pediatr 2023; 182: 1155–1162
13) Levi N, Moravsky G, Weitsman T, et al: A prospective study on myocardial injury after BNT162b2 mRNA COVID-19 fourth dose vaccination in healthy persons. Eur J Heart Fail 2023; 25: 313–318