Coronary artery obstructive lesions following coronary transfer procedures in congenital heart surgery represent one of the most serious complications leading to acute cardiac failure and intractable ventricular arrhythmias; as a result, patients often become dependent on extracorporeal membrane oxygenation (ECMO) in the acute period immediately after cardiac surgery. In the late period after surgery, acute myocardial infarction, sudden cardiac death, and ischemic heart failure requiring eventual heart transplantation can ensue. Immediate evaluation of coronary insufficiency is important to ensure prompt counteraction. In this article, I evaluate surgical coronary revascularization methods and propose modified guidelines for selection of the optimal surgical management.
Frequency and Mortality of Coronary Obstructive Complication
Coronary obstructive complications can occur in many operations requiring coronary transfer procedures and, according to previous reports, the frequency of complications ranges from 5% to 12%.1–3) However, with the advent of coronary transfer techniques, including autologous tissue patch enlargement at the first operation, coronary obstructive complications now occur in 2% to 3% of patients undergoing such procedures.4–6) Coronary revascularization or transfer procedures are performed in various operations, including anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), congenital atresia of the left main trunk, Ross operation in children, and, most importantly, arterial switch operation (ASO) for transposition of the great arteries (TGA) and related abnormalities such as Taussig-Bing anomaly. The most common redo coronary revascularization surgery is required for children with TGA who have undergone ASO in the neonatal period. In addition, many low-birth-weight babies have various abnormalities involving the origin and course of the coronary artery and intramural coronary arteries. Bartoloni et al.7) reported in 2006 that 50% of acute deaths and 100% of late cardiac deaths were due to coronary artery obstruction following ASO. According to recent reports, more than 12% of ASO survivors have some coronary obstructive complications.8, 9) Therefore, in this article, I focus on post-ASO coronary complications.
In Japan, the 30-day mortality rate for 1083 ASO patients collected through the Japan Cardiovascular Surgical Database Congenital Section (JCVS-CS) was 3.2% (35 deaths including ECMO deaths n=24, post-discharge deaths n=1) and the 90-day mortality rate was 5.2% (56 deaths including ECMO deaths n=33, post-discharge deaths n=9).6) An increase in post-discharge sudden death is most likely due to overlooked coronary obstruction.5, 7, 10–12)
Symptomatology and Causes of Coronary Obstruction
Coronary artery obstructive lesions can occur in the early period following coronary transfer procedures; for example, in acute heart failure with difficult weaning from cardiopulmonary bypass or in severe low cardiac output syndrome requiring ECMO.1) In this situation, the coronary obstruction is mostly the result of technical errors or mechanical obstructions secondary to coronary compression, kinking, twisting, and/or stretching. Alternatively, patients who recover from the original surgery may have sudden cardiac death or experience an acute myocardial infarction leading to chronic heart failure that eventually requires heart transplantation long after the original surgery. Late coronary obstruction is mainly caused by a fibromuscular proliferative reaction secondary to an inflammatory process and endothelial dysfunction of the coronary artery.7–10) Late events usually occur within 6 months to 1 year10, 11) after the initial operation but can occur more than 10 years after the ASO.12) For acute episodes, an emergency rescue operation is needed, whereas for late episodes, an urgent or scheduled operation can be planned.6)
Operative Methods and Outcomes
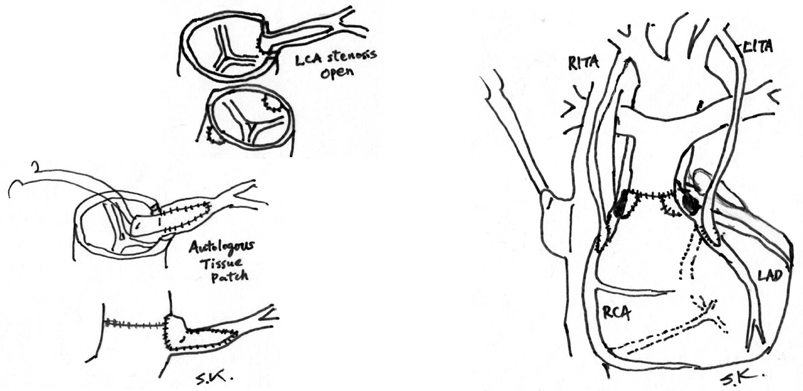
Two different surgical methods have been utilized for the treatment of coronary transfer problems occurring in both the acute and late periods (Fig. 1). One is surgical coronary ostial angioplasty with an autologous tissue patch (SOAP).13–15) Various autologous tissues have been utilized, including autologous saphenous vein, azygous vein, pericardium, and pulmonary arterial wall. The best autologous material for this purpose has not yet been determined, but in the short term, there is no difference in patency or recurrence of stenosis.1, 6) For stenosis or obstruction occurring in the late period, tissue with vascular endothelium may be better, such as an autologous patch obtained from the pulmonary artery or saphenous vein.14) The second method for coronary revascularization is pediatric coronary artery bypass surgery utilizing an internal thoracic artery graft (PCABS-ITA).1, 13, 16–22)
Both methods have comparative advantages and disadvantages, as shown in Table 1. One disadvantage of SOAP is the need for total release of all anastomotic areas in ASO to approach the coronary orifices. This process prolongs the aortic cross-clamp time and cardiopulmonary bypass time, leading to excessive bleeding in critical patients. In contrast, PCABS does not require release of the previous suture line, but the internal thoracic artery and coronary arteries in neonates are small, usually less than 1 mm in diameter. Technical expertise using a surgical microscope may be necessary for anastomosis.21–25)
Table 1 Advantages and disadvantages of 2 surgical methods for the management of coronary obstructive lesions following coronary transfer procedures | Surgical Coronary Ostial Angioplasty (SOAP) | Pediatric CABG with ITA (PCABS) |
|---|
| Approach | Total release of anastomosis of ASO | Dissection unnecessary |
| Aortic cross-clamp time and cardio-pulmonary bypass time | Long | Short |
| Coronary blood flow | Normal, antegrade | Partially retrograde, but no evidence of myocardial injury |
| Advantages and Disadvantages | Direct plasty for lesions, Stenosis recurrence
Acute phase: technical and/or mechanicalcompression, kinking, twisting and/or stretching
Chronic phase: fibromuscular proliferation endothelial dysfunction | Lesions untouching
Small size anastomosis: ITA-coronary artery
String to closure of ITA grafts
Use of a surgical microscope |
| Patch or Graft | Autologous tissue patch (vein or pulmonary artery)
ITA preserved for possible later use | Use of ITA graft
Many other arterial grafts can be used at grown-up age |
| ASO, arterial switch operation; ITA, internal thoracic artery. |
No difference in early survival rates between the 2 methods have been reported, according to American2) and European3) databases, as shown in Tables 2 and 3. An understanding of the comparative long-term outcomes between the 2 redo revascularization methods is very important but such reports have been very limited. In 2018, Thammineni et al.2) reported transplantation-free survival rates following both surgical revascularization methods from an analysis of the Pediatric Cardiac Care Consortium (PCCC) database. According to their report, 20-year transplantation-free Kaplan–Meier survival rates were approximately 75% in the PCABS group and 45% in the SOAP group. However, the case numbers of both groups (n=36 for PCABS; n=31 for SOAP) were small, and the difference between the two groups was not significant (log-rank p=0.28). Further reports of long-term results following pediatric coronary revascularization surgeries are, therefore, very much anticipated.
Table 2 Mortality (no. of cases) following rescue operation in the early postoperative period and urgent or scheduled operation in the late postoperative period| Database | n | Mortality Early emergency rescue operation | Mortality Late scheduled or urgent operation |
|---|
| PCCC*1 (USA) | 123 | 29.8% | 0% |
| 30 (ASO) |
| ECHSA*2 (Europe) | 80 | 32% (11/34) | 3.8% (3/80) |
| 37 (ASO) |
| JCVSD-CS*3 (Japan) | 13 (ASO) | 75% (6/8) | 0% (0/5) |
| ASO, arterial switch operation. *1PCCC: Pediatric Cardiac Care Consortium [Ref. 2]. *2ECHSA: European Congenital Heart Surgeons Association [Ref. 3]. *3JCVSD-CS: Japan Cardiovascular Surgery Database-Congenital Section [Ref. 6]. |
Table 3 Reports from the United States, Europe and Japan databases regarding surgical treatments for coronary obstructive complication following coronary transfer procedures (also see Fig. 1)| Database | Case no. | Age at operation | SOAP | PCABS |
|---|
| Case no. | Age at operation | Case no. | Age at operation |
|---|
| PCCC*1 (USA) (1982∼2011) | n=123 | 4.4 yrs (3 days∼17.4 yrs) | n=51 (41.5%) | 2.6 yrs (5 days∼16.7 yrs) | n=72 (58.5%) | 6.8 yrs (3 days∼17.4 yrs) |
| POST ASO | POST ASO | POST ASO |
| n=30 (27%) | n=11 (37%) | n=19 (63%) |
| ECHSA*2 (Europe) (1973∼2011) | n=80 | 2.3 yrs (2 days∼16.9 yrs) | n=15 (19%) | | n=65 (81%) | |
| POST ASO | POST ASO | POST ASO |
| n=37 (46%) | n=5 (14%) | n=32 (86%) |
| JCVSD*3 (Japan) (2011∼2018) | POST ASO
n=13 | rescue 24 days (4∼234 days) | n=7 (54%) | 40days (6 days∼234 days) | n=6 (46%) | 96 days (4 days∼501 days) |
| urgent 111 days (93∼501 days) | body weight (kg) | 2.7 (2.3∼6.3) | body weight (kg) | 3.9 (2.7∼9.1) |
| POST ASO: PCABS or SOAP for post ASO patients only. *1PCCC: Pediatric Cardiac Care Consortium [Ref. 2]. *2ECHSA: European Congenital Heart Surgeons Association [Ref. 3]. *3JCVSD-CS: Japan Cardiovascular Surgery Database-Congenital Section [Ref. 6]. ASO, Arterial Switch Operation; PCABS, Pediatric Coronary Artery Bypass Surgery; SOAP, Surgical Ostial Angio-Plasty. |
Mortality following a rescue coronary revascularization shortly after the primary ASO surgery is high, ranging from 25% to 75%,2, 3, 6) because of the numerous risk factors present in these low-body-weight neonates. In this situation, many neonates are on ECMO, which is required in 5.7% of patients after ASO, and mortality on ECMO approaches 56%.6) Immediate coronary arterial evaluation is mandatory in patients on ECMO. In contrast, for urgent but scheduled surgery in the late period after ASO, reoperative mortality is very low, ranging from 0 to 3.8% (Table 2).2, 3, 6)
Comparative Analysis of Database Reports from the United States, Europe, and Japan
Before 2000, the incidence of coronary complications following ASO was relatively high10) but recently, with advances in neonatal surgery, it has decreased to 2% to 3%. It is difficult, therefore, to analyze outcomes of many patients in a single center or hospital. For this reason, database analysis is important. At present, reports by PCCC in the United States,2) the European Congenital Heart Surgeons Association (ECHSA),3) and the Japan Cardiovascular Surgical Database, Congenital Section (JCVSD-CD)6) are available.
Reports from PCCC2) and ECHSA3) did not specify post-ASO coronary complications; the numbers of post-ASO patients who subsequently underwent coronary revascularization were 30 (27%) in PCCC and 37 (46%) in ECHSA. In JCVSD, only 13 post-ASO patients (1.2%) underwent redo coronary revascularization.6) This rate is very low compared with other reports2, 3) and suggests that some cases were overlooked because of the high 90-day mortality rate of 5.2% including an increase in post-discharge deaths (9 children) compared to the 30-day mortality of 3.2%. As shown in Table 3, post-ASO coronary complications were managed by PCABS in 63% to 86% of patients and by SOAP in 14% to 37% of patients, according to the PCCC and ECHSA databases.2, 3) Thus, in the United States and Europe, PCABS was more commonly performed than SOAP for the management of post-ASO coronary complications; in Japan, this was reversed (46% PCABS vs 54% SOAP).6) The median age of patients was higher in the PCABS group than in the SOAP group, although the lowest age was not different (3 days for PCABS vs 5 days for SOAP) (Table 3). PCABS has now been performed in many centers and children’s hospitals, and the use of ITA grafts for children is now well established because of the excellent growth potential of the graft in rapidly growing children.19–21, 26–32) In addition, adaptive flow volume capacity of ITA grafts appears bigger and faster than that we previously considered.19–22) Use of a surgical microscope for PCABS in young children is slowly but steadily expanding.21–25)
Importance of Early Detection and Selection of Operative Methods
Making a quick decision is essential for redo coronary revascularization surgery for post-ASO coronary complications. To facilitate this decision, intraoperative coronary arteriography would be very beneficial for validation of coronary anastomosis to avoid overlooked coronary problems that may lead to persisting heart failure after the ASO or sudden death after discharge. When ASO is performed in the hybrid operation room, it would be much easier to perform intraoperative coronary arteriography following the coronary transfer procedure. Coronary angiography can be carried out through a cardioplegic needle with a release of aortic cross-clamping. When coronary anastomotic problems are found, reparative SOAP can be done quickly, with insertion of an autologous tissue patch such as a pulmonary artery slice. In contrast, when coronary problems are found late after the ASO, PCABS with an ITA graft is convenient and does not require any dissection of the original ASO operative areas. In the later period after ASO, fibromuscular proliferative concentric coronary stenosis or obstruction is common caused by inflammation.7–12) Thus, direct plasty (i.e., SOAP with a patch) may aggregate local inflammation and promote stenosis recurrence.1) Bypass surgery (i.e., PCABS-ITA) to the intact area of the coronary artery is recommended in this scenario, unless there are no other complications such as neoaortic dilatation with valve regurgitation or pulmonary stenosis.16)
European guidelines reported in 2017 regarding TGA33) showed that SOAP is considered “recommendation I, evidence level C” or I(C), and PCABS as IIa(C), but there is no description of the selection criteria for these methods. The Japanese guidelines published in 202234) state that coronary obstructive complications can be managed by percutaneous coronary intervention, SOAP, and/or PCABS, but the long-term outcomes of these management methods are unknown. Based on the analysis of the world databases, I propose the following revised guideline criteria (Table 4).
Table 4 Proposal for a new guideline| Post-ASO coronary stenosis and obstruction |
|---|
| Rescue procedure (technical errors or mechanical compression, twisting, kinking and/or stretching) |
| SOAP c̄ Patch | I(C) |
| PCABS c̄ ITA | IIa(C) |
| Late occlusion (fibro-muscular proliferation) |
| PCABS c̄ ITA | I(C) |
| SOAP c̄ Patch | IIa(C) |
| Level of stenosis |
| LMT≤75%, localized at orifice | |
| SOAP c̄ Patch | I(C) |
| LMT>90%, extending to bifurcation | |
| PCABS c̄ ITA | I(C) |
In a rescue operation shortly after ASO, coronary insufficiency is mostly caused by technical or mechanical coronary obstructions such as compression, kinking, twisting, or stretching, so that SOAP is regarded as I(C) and PCABS as IIa(C), as recommended by the European guideline.33) However, in the later period after the ASO, coronary obstruction is mainly due to fibromuscular proliferative inflammation associated with endothelial dysfunction; then, PCABS will be I(C) and SOAP will be IIa(C) because of the lesser invasiveness of PCABS-ITA associated with the lesser chance for recurrence of fibroproliferative stenosis. Simultaneous combined use of both surgical revascularization procedures as a fail-safe addition should be limited as indicated III(C) in the European guideline33) because of the coronary flow competition often leading to the string phenomenon of the ITA graft.
In addition, stenosis >90% or total obstruction extending to the bifurcation area of the left main coronary artery favors PCABS, whereas stenosis <75% to 90% not extending into the bifurcation area favors SOAP. This proposal for a new guideline must be validated in the future by evidence accumulated from a large number of cases.
In conclusion, long-term (>20 year) patency of the coronary artery or an event-free survival rate is important to establish new guidelines for selection of coronary revascularization procedures. A hybrid operating room may be beneficial in detecting coronary anastomotic mishaps as promptly as possible, and the use of a surgical microscope for fine vessel anastomosis will add a new tool for pediatric cardiac surgeons. I certainly welcome the opinions for and/or against my proposals for possible new guidelines.
Conflicts of Interest
The author has no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Note
This article is based on a study first reported in Pediatric Cardiology and Cardiar Surgery 2023; 39(1): 3–8 (in Japanese).
引用文献References
1) Kitamura S: Pediatric coronary artery bypass surgery for congenital heart disease. Ann Thorac Surg 2018; 106: 1570–1577
2) Thammineni K, Vinocur JM, Harvey B, et al: Outcomes after surgical coronary artery revascularization in children with congenital heart disease (Pediatric Cardiac Care Consortium Report). Heart 2018; 104: 1417–1423
3) Vida VL, Torregrossa G, De Franceschi M, et al; European Congenital Heart Surgeons Association (ECHSA): Pediatric coronary artery revascularization: A European multicenter study. Ann Thorac Surg 2013; 96: 898–903
4) Fricke TA, Bell D, Daley M, et al: The influence of coronary artery anatomy on mortality after the arterial switch operation. J Thorac Cardiovasc Surg 2020; 160: 191–199
5) van Wijk SWH, van der Slelt F, ter Heide H, et al: Sudden death due to coronary artery lesions long-term after the arterial switch operation: A systematic review. Can J Cardiol 2017; 33: 1180–1187
6) Kitamrua S, Tachimori H, Murakami A, et al: Mortality analysis of arterial switch operation for transposition of the great arteries with and without ventricular septal defect. Eur J Cardiothorac Surg 2022; 61: 797–804
7) Bartoloni G, Bianca S, Patene L, et al: Pathology of coronary narrowing after arterial switch operation: Autopsy findings in two patients who died within 3 months of surgical treatment and review of the literature. Cardiovasc Pathol 2006; 15: 49–54
8) Tsuda T, Bhat AM, Robinson BW, et al: Coronary artery problems late after arterial switch operation for transposition of the great arteries. Circ J 2015; 79: 2372–2379
9) Bonhoeffer P, Bonnet D, Piechaud JF, et al: Coronary artery obstruction after the arterial switch operation for transpotsition of the great arteries in newbors. J Am Coll Cardiol 1997; 29: 202–206
10) Tsuda E, Imakita M, Yagihara T, et al: Late death after arterial switch operation for transposition of the great arteries. Am Heart J 1992; 124: 1551–1557
11) Jemmali M, Marini D, Calcagni G, et al: Pediatric coronary artery bypass after arterial switch operation: Noninvasive evaluation with ECG-gated 64 slice CT with routine practice. Ann Thorac Surg 2007; 84: 1398–1399
12) Singh DP, Menon S: Coronary issues following arterial switch operation: Are we missing it? J Am Coll Cardiol 2019; 73: 2767
13) Koubský K, Gebauer R, Tláskal T, et al: Long-term survival and freedom from coronary artery reintervention after arterial switch operation for transposition of the great arteries: A population-based nationwide study. J Am Heart Assoc 2021; 10: e020479
14) Krokovay A, Pretre R, Kretschmar O, et al: Anatomical reconstruction of proximal coronary artery stenosis in children. Eur J Cardiothorac Surg 2022; 62: ezac302
15) Bergoënd E, Raisky O, Degandt A, et al: Myocardial revascularization in infants and children by means of coronary artery proximal patch arteris-plasty or bypass grafting: A single-institional experience. J Thorac Cardiovasc Surg 2008; 136: 298–306
16) Kitamura S: Direct plasty or bypass? That is still a question. (Invited Commentary). Eur J Cardiothorac Surg 2022; 62: ezac323
17) Ebels T, Meuzelaar K, Gallendat-Huet RC, et al: Neonatal arterial switch operation complicated by intramural left coronary artery and treated by left internal mammary artery bypass graft. J Thorac Cardiovasc Surg 1989; 97: 473–475
18) Rheuben KS, Kron IL, Bulatovic A: Internal mammary artery bypass after the arterial switch operation. Ann Thorac Surg 1990; 50: 125–126
19) Hohri Y, Yamagishi M, Maeda Y, et al: Coronary artery bypass grafting for coronary artery anomalies in infants and young children. Interact Cardiovasc Thorac Surg 2022; 35: ivacc119
20) Nair KKS, Chan KC, Hickery MSJ: Arterial switch operation: Successful bilateral internal thoracic artery grafting. Ann Thorac Surg 2000; 69: 949–951
21) Kitamura S: A new arena in cardiac surgery: Pediatric coronary artery bypass surgery. Proc Jpn Acad, Ser B, Phys Biol Sci 2018; 94: 1–19
22) Iwata Y, Takeuchi T, Konuma T, et al: Infant coronary bypass grafting completely under surgical microscope. JTCVS Tech 2021; 10: 441–443
23) Kitamura S: Commentary: Try it, you may like it. JTCVS Tech 2021; 10: 444–445
24) Burkhart HM, Nakamura Y, Mir A: Commentary: Arterial switch operation; it’s the little things that count. JTCVS Tech 2021; 10: 446–447
25) Catapano J, Zukar R, Honjo O, et al: Microvascular coronary artery repair and grafting in infancy and early childhood. Oper Tech Thorac Cardiovasc Surg 2015; 20: 148–161
26) Kitamura S, Seki T, Kawachi K, et al: Excellent patency and growth potential of internal mammary artery grafts in pediatric coronary artery bypass surgery: New evidence for a “Live” conduit. Circulation 1988; 78: I-129–I-139
27) Mavroudis C, Backer C, Duffy E, et al: Pediatric coronary artery bypass for Kawasaki, congenital, post arterial switch and iatrogenic lesions. Ann Thorac Surg 1999; 68: 506–512
28) Legendre A, Chantepie A, Belli E, et al: Outcome of coronary artery bypass grafting performed in young children. J Thorac Cardiovasc Surg 2010; 139: 349–353
29) Viola N, Alghamdi AA, Al-Radi OO, et al: Midterm outcomes of myocardial revascularization in children. J Thorac Cardiovasc Surg 2010; 139: 333–338
30) Ramirez-Marroquin SE, Iturriaga-Hernández A, Calderón-Colmenero J, et al: Coronary revascularization in children at a Mexican Cardiac Center: Thirteen-year outcomes. World J Pediatr Congenit Heart Surg 2017; 8: 600–604
31) Arnaz A, Sarioglu T, Yalcinbas Y, et al: Coronary artery bypass grafting in children. J Card Surg 2018; 33: 29–34
32) Lazar HL: Coronary artery revascularization in infants, children, and adolescents: The internal mammary artery is still the conduit of choice. J Card Surg 2018; 33: 35
33) Sarris GE, Balmer C, Bonou P, et al: Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum. Eur J Cardiothorac Surg 2017; 51: e1–e32
34) Ohuchi H, Kawada M, Akagi T, et al: 8.2.3 Coronary obstruction and Stenosis, in JCS 2022 Guideline on Management and Re-interventional Therapy in Patients with Congenital Heart Disease Long-Term after Initial Repair, pp80–82