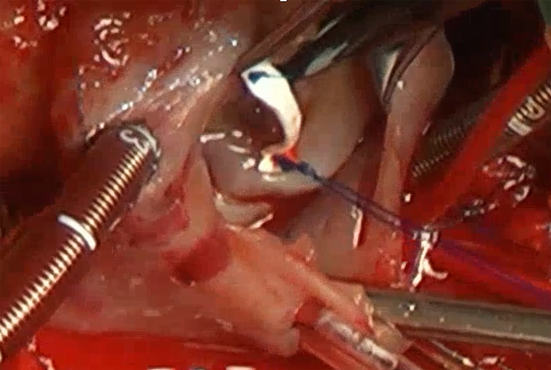
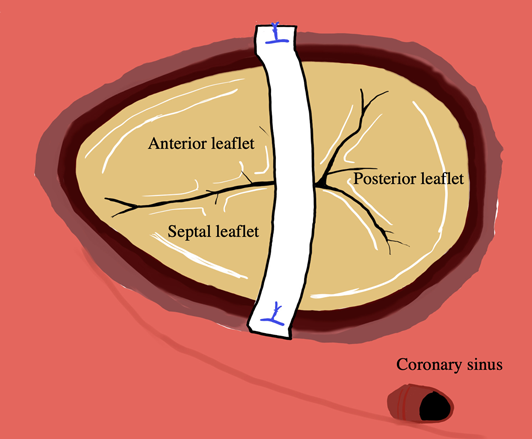
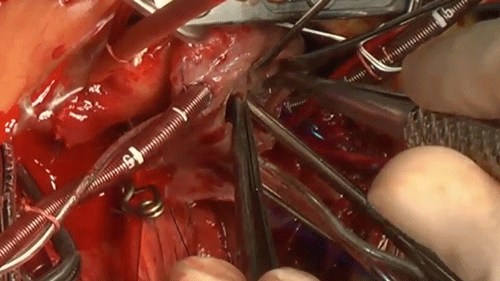
Tricuspid Valve Repair with a Bridging Technique Across the Valve for Tricuspid Stenosis and Regurgitation at the Norwood Procedure
Takuya Matsuzawa 1,2,Naoki Wada1,Risa Shinbori1,Tubasa Furuya1,Yuya Komori1,Yuta Kuwahara1,Masatoshi Shimada1,Yukihiro Takahashi1Takuya Matsuzawa
1,2,Naoki Wada1,Risa Shinbori1,Tubasa Furuya1,Yuya Komori1,Yuta Kuwahara1,Masatoshi Shimada1,Yukihiro Takahashi1Takuya Matsuzawa 1,2, Naoki Wada1, Risa Shinbori1, Tubasa Furuya1, Yuya Komori1, Yuta Kuwahara1, Masatoshi Shimada1, Yukihiro Takahashi1
1,2, Naoki Wada1, Risa Shinbori1, Tubasa Furuya1, Yuya Komori1, Yuta Kuwahara1, Masatoshi Shimada1, Yukihiro Takahashi1 1 Department of Pediatric Cardiovascular Surgery, Sakakibara Heart Institute ◇ Tokyo, Japan
2 Department of Cardiovascular Surgery, Teikyo University ◇ Tokyo, Japan
受付日:2024年2月27日Received: February 27, 2024
受理日:2024年5月16日Accepted: May 16, 2024
発行日:2025年1月31日Published: January 31, 2025