It is challenging to manage severe aortic stenosis (AS) in young patients with significant intellectual disability. Aortic valve replacement (AVR) using a mechanical valve requires life-long anticoagulation, which is not deemed feasible in these patients. A bioprosthetic valve can be used, although it has limited durability. On the other hand, bioprosthetic AVR could be followed by valve-in-valve transcatheter AVR (TAVR) in the future when prosthetic re-replacement should become necessary.
One of the major complications of valve-in-valve TAVR is coronary flow obstruction, which is commonly caused by direct or nearly-direct contact between a bioprosthetic valve leaflet and a coronary ostium. Risk factors for coronary flow obstruction include placement of the bioprosthetic valve at a supra-annular position, as well as a narrow sino-tubular junction. In patients with AS and a small aortic annulus, the Konno procedure can be used to enlarge the annulus anteriorly, which provides an enlarged sinus of Valsalva and an increased distance between the coronary ostia and the bioprosthetic valve leaflets; this geometric modification reducing the risk of coronary flow obstruction. We report a young woman with congenital AS and ring chromosome 18 syndrome who underwent bioprosthetic AVR in conjunction with the Konno procedure, by which we anticipated potential valve-in-valve TAVR in the future.
A 25-year-old woman with ring chromosome 18 syndrome, intellectual disability, chronic thyroiditis and goiter, microcephaly, and dwarfism was referred for evaluation of congenital AS. At 10 years of age, she had undergone aortic commissurotomy. Subsequently, the AS and aortic regurgitation (AR) progressed, which required the second surgery for commissurotomy and aortic valvuloplasty using the fresh autologous pericardium 5 years later. When we evaluated her this time, her AS and AR were of a severe degree, and she was symptomatic (New York Hear Associate classification II–III). Another surgical intervention was therefore planned.
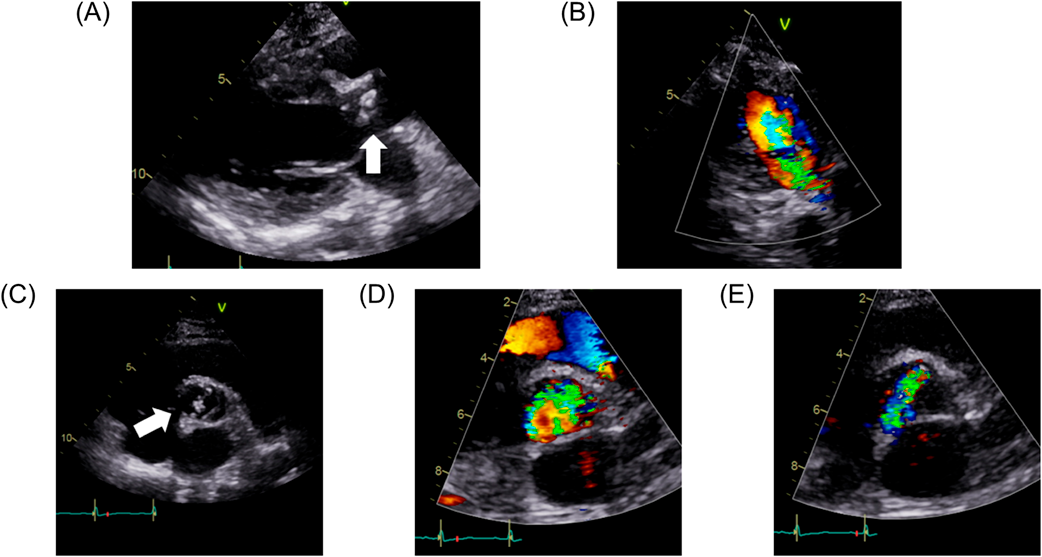
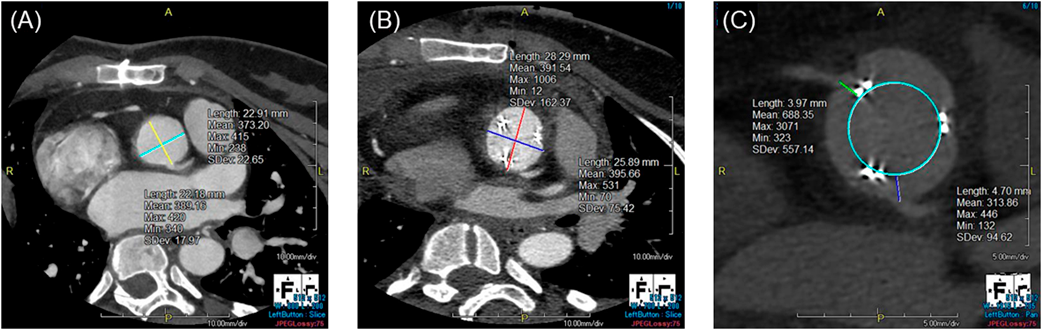
At the time of surgery, the patient was 135 cm in height and weighed 38.0 kg. Chest X-ray showed cardiomegaly and pulmonary venous congestion. Brain nitric peptide level was 66.8 pg/mL. Echocardiography (Fig. 1) showed a left ventricular end-diastolic diameter of 43 mm, left ventricular ejection fraction of 67%, severe AS (aortic valve area, 0.72 cm2), peak velocity of 4.6 m/s, peak pressure gradient of 84 mmHg, and severe AR. The pulmonary valve was normal in diameter with no pulmonary regurgitation noted. On contrast-enhanced computed tomography (CT), diameters of the aortic annulus, the sinus of Valsalva, the sino-tubular junction, and the ascending aorta were 19.0 mm, 22.9 mm×22.2 mm, 19.3 mm×22.7 mm, and 28.3 mm, respectively. The right and the left coronary arteries took off 15.5 mm and 15.1 mm above the ventriculo-arterial junction, respectively.
Given the narrow aortic annulus, mechanical AVR was initially considered; however, this was eventually declined because it would have required the patient to take oral medication indefinitely after surgery, which did not appear feasible considering her significant intellectual disability. We therefore decided to choose bioprosthetic AVR in conjunction with the Konno procedure. Consent for the surgery was obtained from the patient’s parents.
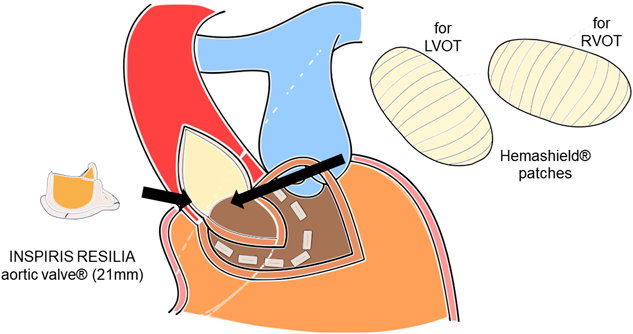
Through the third median sternotomy, severe adhesions were carefully dissected and bi-caval cardiopulmonary bypass was established. Under cardiac arrest, the anterior aspect of the ascending aorta was incised longitudinally. The orifice of the right coronary artery was confirmed, and the incision was extended into the right coronary sinus to the left of the right coronary arterial ostium, close to the right-left commissure. Then, a transverse incision was placed along the free wall of the right ventricular outflow tract 1 cm below the pulmonary valve. The ventriculo-infundibular fold and aortic annulus were then divided between the incisions to the aortic wall and the right ventricular free wall. The incision was extended into the interventricular septum 7 mm under the pulmonary valve. A patch molded from a 24 mm Hemashield tube graft (Getinge, Rastatt, Germany) was first fixed to the ventricular septum using pledgeted sutures. Pledgeted sutures were also placed along the aortic annulus on the Hemashield patch, and a 21 mm Inspiris Resilia aortic valve (Edwards Lifesciences, Irvine, CA, USA) was inserted in the enlarged aortic annulus. The Hemashield patch was then sutured to the ascending aorta using running stitches. Another Hemashield patch was also attached for the anterior aspect of the right ventricle using running stitches. The operative schema is shown in Fig. 2.
Her postoperative course was uneventful. She was extubated on the postoperative day 2, discharged from the intensive care unit on the day 5, and discharged from the hospital on the day 11. Postoperative echocardiography showed that peak velocity and peak pressure gradient had decreased to 2.30 m/s and 21.2 mmHg, respectively. On contrast-enhanced CT, diameters of the aortic annulus, the sinus of Valsalva, and the sino-tubular junction had been enlarged to 25.2 mm, 28.3 mm×25.9 mm, and 22.4 mm×24.6 mm, respectively. The distances between the bioprosthetic valve leaflets and the right and the left coronary ostia were 4.0 mm and 4.7 mm, respectively. Fig. 3 shows the sinus of Valsalva on contrast-enhanced CT before and after the surgery.
In this case report, we described a 25-year-old patient with severe AS/AR, small aortic valve annulus, and intellectual disability associated with ring 18 chromosome. According to the report by Carter E et al., congenital heart defects commonly associated with this particular chromosomal abnormality are pulmonary stenosis and atrial septal defects.1) In addition, it has been reported by van Trier DC et al. that AS was detected in 16% of patients with the 18q deletion syndrome.2) While data on the longevity of ring 18 chromosome is limited due to its rarity, Soileau et al. reported a patient with ring 18 chromosome who died at age 48.3)
Bioprosthetic AVR relieves patients from the necessity of anticoagulation therapy, but its durability is limited to 10–15 years. The so-called valve-in-valve TAVR has been developed as an alternative to surgical reoperation in patients who are at high risk for open heart surgery for bioprosthetic valve failure.4) This patient is considered suitable for this strategy, because of multiple re-sternotomies and the chromosomal abnormality. These two factors are not taken into account by currently used risk assessment scores (JapanSCORE, EuroSCORE II or STS score), which complicates the assessment of risk of open-heart surgery in our case.
As for the size and the type of a bioprosthetic valve, we used 21 mm INSPIRIS RESILIA valve®. One can argue that a larger-sized valve could have been selected and would be more advantageous for future TAVR. However, a larger-sized valve can lead to smaller distances between the bioprosthetic valve leaflets and the coronary arterial ostia, increasing the risk of coronary orificial obstruction after valve-in-valve TAVR. In our case, the valve-to-coronary arterial distances were 4.0 mm for the right coronary ostium and 4.7 mm for the left coronary ostium on postoperative CT. This result indicates that our selection of the valve size was reasonable, considering the fact that the valve-to-coronary arterial distance less than 4.0 mm has been reported to be a risk for coronary orificial obstruction.5) INSPIRIS RESILIA valve is designed to dilate its valve annulus at TAVR. The annulus-dilatable design of INSPIRIS was demonstrated in the previous report from our department.6) Based on the patient’s body size, the 21 mm INSPIRIS RESILIA valve could allow TAVR twice eventually.
Our patient had a small aortic annulus which required aortic annular enlargement in order to implant a bioprosthetic valve. Recently, Yang et al. reported a method of enlarging the aortic root which uses a Y-incision and a patch between the left coronary sinus and the noncoronary sinus.7) Although this technique provides sufficient aortic annulus enlargement, it is questionable whether it increases the distances between the bioprosthetic valve leaflets and the coronary ostia. Therefore, we would prefer to perform the Konno procedure, which expands the right coronary sinus and would reduce the risk of developing coronary flow obstruction after future valve-in-valve TAVR.
Other than bioprosthetic AVR, we have two surgical options when treating patients with severe AS who cannot be anticoagulated after surgery; the Ross procedure and the Ozaki procedure (aortic valve neocuspidization). The Ross procedure uses pulmonary valve autograft to replace the aortic valve, which offers growth potential and superior hemodynamics compared with prosthetic valves. As for the Ozaki procedure, the diseased native leaflets are completely excised, and new ones are constructed using the pericardium. Given the narrow aortic root structure, annular enlargement would have been necessary to achieve a sufficient orifice area of the valve and to prevent AS in the longer term in any of the procedures, including the Ross procedure, the Ozaki procedure, and bioprosthetic AVR. Either the Ross or Ozaki procedures can be used in patients with a small aortic annulus even when annular enlargement is performed at the same time.8) A couple of disadvantages of the Ross-Konno procedure are late onset of aortic root dilatation and right ventricular outflow tract dysfunction. Schneider et al. reported that freedom from such right heart problems was 40% and that freedom from autograft reoperation at 15 years was 57%, respectively.9) Moreover, patients with preoperative AR who undergo the Ross procedure may experience autograft dilatation.10) These data, and uncertain clinical course associated with ring chromosome 18 syndrome, seemed to make parents to hesitate the Ross procedure and they preferred a bioprosthetic AVR. Aortic valve reconstruction using the Ozaki procedure was another alternative that has been reported to have favorable midterm outcomes11); at 118 months, the cumulative incidence of reoperation and recurrent AR moderate or greater was 4.2%, and 7.3%, respectively. Acceptable short-term outcomes have been reported in children with congenital aortic and truncal disease who underwent the Ozaki procedure.12) In our case, nonetheless, we did not choose this strategy because of the following reasons. First, this procedure involves reconstructing the aortic valve with three separate pieces of the autologous pericardium. This was not practical; severe adhesion following multiple previous surgery would have inhibited harvest of the autologous pericardium. The only patch material available in Japan is glutaraldehyde-fixed bovine pericardium, which is likely to calcify after implantation. Second, the long-term outcomes with this procedure, especially in chromosomal disorder, are not well-established.