Circumferential calcification in the left ventricular myocardium is often described as a consequence of myocarditis.1–5) It has never been reported, on the other hand, that such calcification was seen during early perioperative period of pediatric open heart surgery. Herein we report a patient who underwent the Yasui operation in a stage way and then developed fatal biventricular diastolic dysfunction postoperatively. We retrospectively considered that left ventricular failure was due to whole circumferential myocardial calcification around the left ventricle.
The patient was female, born at 38 gestational weeks with body weight of 2260 g. After birth, she was diagnosed with type B interrupted aortic arch, perimembranous type ventricular septal defect (VSD), small aortic valve annulus (aortic valve diameter Z-score of −3.5), persistent ductus arteriosus, patent foramen ovale, and 22q.11.2 microdeletion. She underwent hybrid stage I palliation consisting of bilateral pulmonary artery banding and ductal stenting at the age of 5 days. Cardiac catheter examination at the age of 5 months revealed that left and right ventricular end-diastolic volumes were 83% and 129% of anticipated normal values, respectively, and pulmonary-to-systemic blood flow ratio was 0.65 (Table 1). At 9 months old weighing 6.1 kg, she underwent the Yasui operation involving the Norwood-type arch reconstruction with glutaraldehyde treated autologous pericardial patch, and the Rastelli procedure consisting of creation of an interventricular tunnel from the left ventricle to the neo-aortic (morphologically pulmonary) valve via VSD and insertion of a right ventricle-to-pulmonary artery conduit with a 14 mm-diameter expanded polytetrafluoroethylene graft containing a handmade trileaflet valve. Cardiopulmonary bypass time during the Yasui operation was 564 minutes, and the aortic cross-clamp time was 158 minutes.
Table 1 Summary of cardiac catheterization data over the course of treatments | before Yasui op
(5 months old) | after Yasui op
(21 days after Yasui op) | after Yasui op takedown
(51 days after Yasui op) |
|---|
| Cardiac Index (L/min/m2) | 8.52 | 3.19 | 2.19 |
| Qp/Qs | 0.65 | 2.1 | 1 |
| LVEDV (% of normal) | 83 | 111 | 133 |
| RVEDV (% of normal) | 129 | 110 | 181 |
| LVEF (%) | 45 | 46 | 23 |
| RVEF (%) | 58 | 36 | 29 |
| SVCP (mmHg) | 4 | 15 | 18 |
| LVEDP (mmHg) | 7 | 19 | 29 |
| RVEDP (mmHg) | 7 | 14 | 29 |
| Mean PAP (mmHg) | NA | 15 | NA |
| NA, not available; LVEDP, left ventricular end-diastolic pressure; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; PAP, pulmonary arterial pressure; Qp/Qs, pulmonary-to-systemic blood flow ratio; RVEDP, right ventricular end-diastolic pressure; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; SVCP, superior vena cava pressure; Yasui op, the Yasui operation |
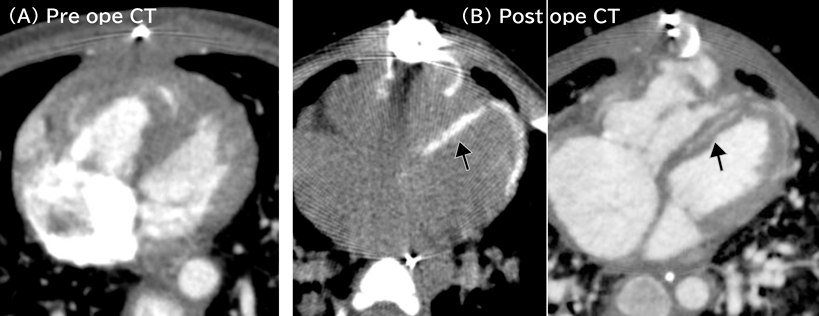
Acute postoperative course was unstable mainly due to right ventricular diastolic dysfunction. Central venous pressure, however, decreased from 20 mmHg to 13 mmHg, so that chest was closed 6 days later. Her hemodynamic condition gradually improved thereafter. We intended to arrange endotracheal extubation, but lactic acidosis and peripheral edema recurred. Cardiac computed tomography scanning at 16 days after the operation showed no aortic arch obstruction, no left ventricular outflow tract obstruction, mild stenoses of the bilateral branch pulmonary arteries, and circumferential myocardial calcification around the left ventricle (Fig. 1). Cardiac catheter examination at 21 days after the operation showed that left and right ventricular end-diastolic pressures were 19 mmHg and 14 mmHg, respectively (Table 1). Pulmonary-to-systemic blood flow ratio was 2.1 due to residual VSD. Therefore, removal of the intraventricular tunnel and downsizing of the right ventricle-to-pulmonary artery conduit were carried out on the day 33 after the Yasui operation. The chest was closed 3 days later when central venous pressure decreased to 10 mmHg. Since then, her hemodynamic condition had rather deteriorated. Cardiac catheter examination 18 days after the takedown revision revealed that both left and right ventricular end-diastolic pressures were equally 29 mmHg (Table 1).
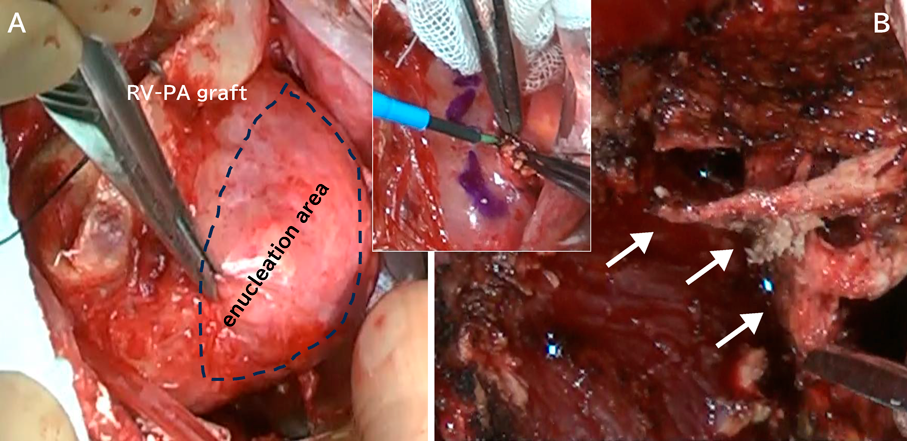
Twenty-two days after the revision, the right ventricle-to-pulmonary artery conduit was removed, and a systemic-to-pulmonary shunt was constructed with a 4 mm expanded polytetrafluoroethylene graft. We partially resected the calcified tissue within the left ventricular wall and submitted the specimen for histopathological examination (Fig. 2). Weaning off from cardiopulmonary bypass was unsuccessful in the operating theater, and the patient returned to the intensive care unit on extracorporeal membrane oxygenation support. The mechanical support was weaned off on the 13 days after the final surgical procedure, but she died 6 days later. Postmortem examination was declined.
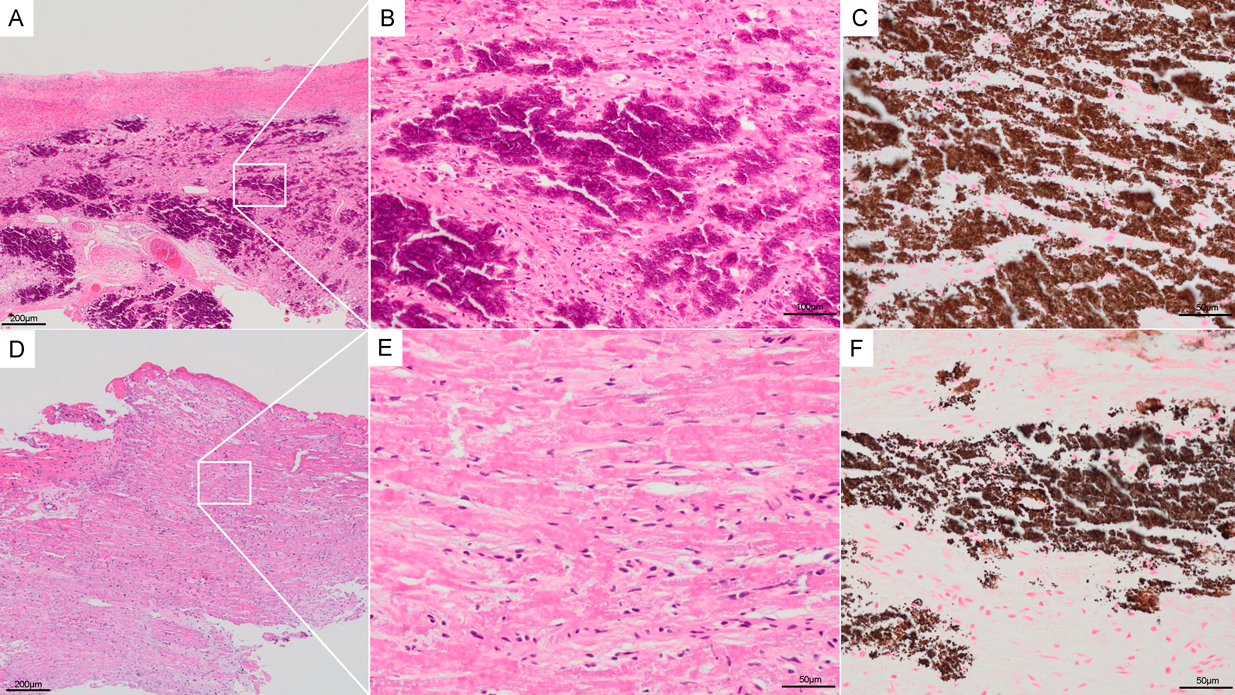
Pathological analysis revealed pathological changes in both myocardial and epicardial tissues (Fig. 3). The myocardium showed extensive calcification, confirmed by von Kossa staining as granular brown deposits throughout the tissue. H&E staining demonstrated widespread degenerative changes in cardiomyocytes with surrounding fibrosis, and scattered inflammatory cell infiltration was observed. Only a small amount of viable myocardial cells remained, indicating severe tissue damage.
The epicardial tissue also exhibited significant pathological changes. H&E staining revealed diffuse calcification accompanied by inflammatory cell infiltration and fibrosis. Von Kossa staining confirmed the presence of scattered calcium deposits in the epicardium, though less extensive compared to the myocardium. These findings suggest that the calcification process affected both tissue layers, contributing to the severe biventricular diastolic dysfunction observed clinically in this patient.
A volume of the right ventricular cavity is often reducing in patients who are candidates for biventricular repair by means of the Rastelli procedure and have previously undergone palliative operations, due to decreased ventricular preload (related to the amount of pulmonary blood flow) and increased right ventricular hypertrophy. In fact, operative course during the acute phase in our patient was complicated by right ventricular diastolic dysfunction. We thought, therefore, hemodynamic instability around 2 weeks after the Yasui operation was derived from insufficient volume for effective right ventricular function. In this respect, we speculated that left ventricular diastolic dysfunction was secondary to residual VSD. We also suspected that placement of a large right ventricle-to-pulmonary artery conduit restricted right ventricular relaxation.
On the contrary, biventricular diastolic dysfunction did not improve after takedown surgery to previous shunt physiology. Rather, the second catheter examination confirmed further deterioration of biventricular diastolic function. We finally came to realize that the myocardial calcified layer within the left ventricular wall was responsible for the intractable circumstance. Right ventricular diastolic dysfunction was presumably caused by calcification within the interventricular septum.
We did not pay much attention to this calcification initially as quite serious. That is why takedown from the complete biventricular circulation to shunt physiology was selected. Entire removal of the calcification within the left ventricular wall appeared unfeasible. Instead of these, mechanical circulatory support until active myocarditis settles could have been an alternative treatment. Indeed, several previous reports described that late improvement of hemodynamic status was confirmed even though once whole circumferential calcification of the left ventricle occurred.1, 2, 6)
The reason why whole circumferential calcification progressed during the perioperative period in this infant is unclear. Although prolonged cardiopulmonary bypass triggers myocardial inflammatory change,7) the artificial circulation usually causes myocardial edema, not calcification. Cardiopulmonary bypass time during the Yasui operation was 564 minutes, and the aortic cross-clamp time was 158 minutes. Some articles discussed that the mechanism of calcification of the left ventricular myocardium would be a metabolic change.1, 2, 6–8) Although our patient had 22q11.2 microdeletion syndrome which was known to associate with hypoparathyroidism, serum calcium was adjusted to maintain the level within a range of 8.6–10.5 mg/dL (corresponding to the ionized calcium level of 1.05–1.31 mEq/L). We did not perform tests for hypoparathyroidism, but in our literature review we found no reports of myocardial calcification caused by excessive calcium supplementation in children with 22q11.2 deletion syndrome. No calcification was observed in any other areas besides the left ventricular myocardium.
As another potential reason for progressive calcification, we noted that she had experienced viral infection 1 month before the Yasui operation. No symptom, nonetheless, remained at the time of readmission for this operation.3)
Furthermore, existing literature provides crucial insights into the mechanisms of onset and progress of myocardial calcification. Particularly, progressive myocardial calcification following myocarditis may involve chronic inflammatory responses and cellular injury, which could be relevant in our case as well.3, 8) Additionally, myocardial ischemia and inflammatory responses associated with cardiopulmonary bypass are significant contributors to myocardial calcification, potentially accelerating myocardial apoptosis and fibrosis.5, 7) Still, it is unprecedented to recognize formation of extensive circumferential myocardial calcification or its rapid development during the early postoperative period as seen in our patient. No established treatments have been documented in the literature.
At first, we focused on diastolic dysfunction based on elevated end-diastolic pressures. We did not perform detailed echocardiographic studies of diastolic function over time. Our assessment mainly came from catheter data. The final catheterization showed both left and right ventricular end-diastolic volumes were much larger than those before surgery and ventricular ejection fractions were very low (Table 1). This suggests that the problem became more complex over time. We now do not consider that her hemodynamic deterioration can be explained by diastolic dysfunction alone. Seemingly the heart had both diastolic and systolic dysfunction in the end. The initial diastolic issues likely led to further complications affecting overall heart function.
In summary, whole circumferential myocardial calcification of the left ventricle occurred after the staged Yasui operation in an infant, which caused lethal biventricular diastolic dysfunction. Hemodynamic instability initially resembled right ventricular diastolic failure due to a small right ventricular cavity after complex biventricular repair. The etiology remained unclear. Takedown from complete biventricular circulation to single ventricular physiology was ineffective to improve overall hemodynamic status.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Consent for Publication
The patient’s parent permitted the use of her personal data for the development of medical science (27/03/2023).
Author Contribution
AH wrote the manuscript (original draft) and contributed to the creation of the figure and table. TH and TS supervised and authorized this study. TN, HN, YF and YI performed the surgery and/or perioperative patient management. All authors read and approved the manuscript.
引用文献References
1) Lippolis A, Buzzi MP, Romano IJ, et al: Stone heart: An unusual case of heart failure with preserved ejection fraction due to massive myocardial calcification. J Cardiol Cases 2021; 23: 145–148
2) Washino M, Tanaka T, Nakase Y, et al: A rare case of myocardial calcification secondary to acute myocarditis due to an Escherichia coli infection. Nagoya J Med Sci 2020; 82: 775–781
3) Wittekind SG, Allen CC, Jefferies JL, et al: Neonatal enterovirus myocarditis with severe dystrophic calcification: Novel treatment with pocapavir. J Investig Med High Impact Case Rep 2017; 5: 1–4
4) Galati G, Leone O, Pasquale F, et al: Histological and histometric characterization of myocardial fibrosis in end-stage hypertrophic cardiomyopathy: A clinical-pathological study of 30 explanted hearts. Circ Heart Fail 2016; 9: e003090
5) Aras D, Topaloglu S, Demirkan B, et al: Porcelain heart: A case of massive myocardial calcification. Int J Cardiovasc Imaging 2006; 22: 123–126
6) Sui M-L, Wu C-J, Yang Y-D, et al: Extensive myocardial calcification in critically ill patients receiving extracorporeal membrane oxygenation: A case report. World J Clin Cases 2022; 10: 4214–4219
7) Anselmi A, Abbate A, Girola F, et al: Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: A review of evidence. Eur J Cardiothorac Surg 2004; 25: 304–311
8) Na J-Y: A heart of stone: An autopsy case of massive myocardial calcification. Forensic Sci Med Pathol 2018; 14: 102–105