Tricuspid valve (TV) dysplasia is a rare congenital cardiac malformation with clinical manifestations similar to those of Ebstein disease.1) In recent years, TV dysplasia is frequently diagnosed during early fetal life. Severe TV dysplasia with gross cardiomegaly is often associated with pulmonary hypoplasia and has high perinatal morbidity and mortality.1, 2) Neonates in profound cardiogenic shock caused by severe TV dysplasia or Ebstein malformation can be salvaged with fenestrated right ventricular exclusion and systemic to pulmonary shunt (the so-called modified Starnes procedure). Although the procedure has improved the survival rate in patients with such serious problems, pulmonary hypoplasia and atelectasis caused by severe cardiac enlargement during the fetal stage can result in poor ventilation and make postoperative management difficult.3, 4)
We report a neonate with TV dysplasia and pulmonary atresia who underwent right ventricular exclusion on the day of birth. We used veno-venous (VV) extracorporeal membrane oxygenation (ECMO) support for early postoperative pulmonary dysfunction. We also performed high-frequency ventilation (HFV) and surfactant therapy for respiratory management, resulting in improved pulmonary function and a good outcome.
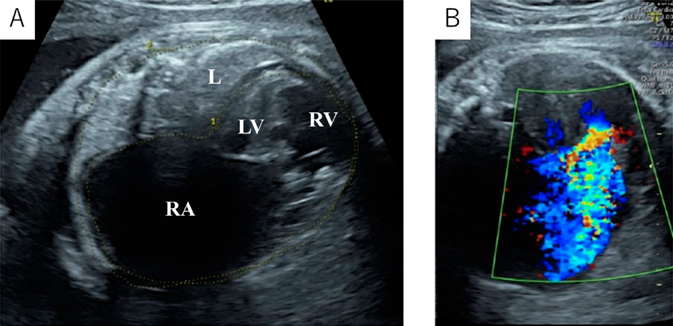
A 39-year-old gravida (gravida 3, para 0) was referred to our hospital at 24 weeks’ gestation for the perinatal care of her fetus diagnosed as having severe tricuspid valve regurgitation. A fetal echocardiography revealed severe tricuspid regurgitation, anatomically atretic pulmonary valve, and ductus arteriosus with retrograde blood flow. The tricuspid valve was severely dysplastic and thickened without typical plastering, with its diameter of 8.9 mm (z-score: 1.31). Pericardial effusion was noted. The cardiothoracic area ratio (CTAR) and Celemajer index2) were 0.46 and 0.9, respectively. After 33 weeks’ gestation, the right atrium and the right ventricle gradually dilated. The right ventricle was thin, and the left ventricle was banana-shaped. At 36 weeks’ gestation, her CTAR and Celemajer index increased to 0.68 and 1.6, respectively (Fig. 1). The tricuspid valve annular diameter was 17.0 mm (Z-score: 1.94) and the tricuspid regurgitation jet peak velocity was 2.3 m/s. The Great Ormond Street Echocardiography (GOSE) score,5) Simpson Andrews Sharland (SAS) score,6) and Tricuspid malformation Prognosis Prediction (TRIPP) score7) were IV, 7, and 5, respectively. The predicted mortality rate from the GOSE score was 100%, the SAS score also predicted high mortality, and the TRIPP score was borderline.5–7) Based on these fetal echocardiographic findings, we predicted that the baby would be in a critical condition after birth due to severe tricuspid regurgitation and pulmonary insufficiency. Therefore, we planned an elective cesarean section and prepared for early cardiac surgery.
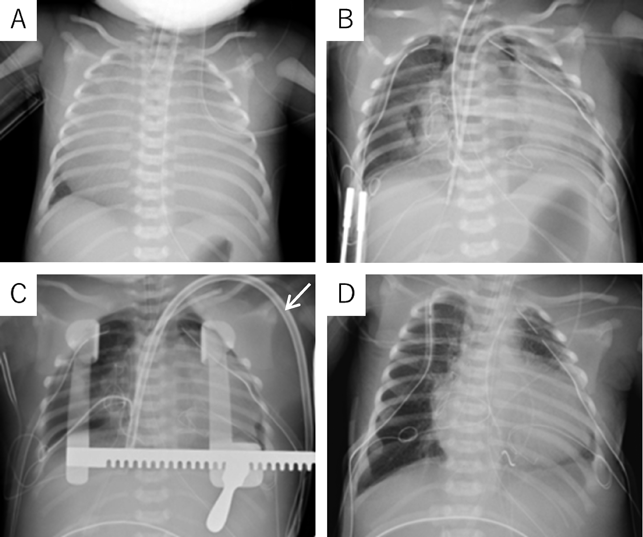
At 37 weeks 5 days’ gestation, a 2,756 g female baby was delivered via elective cesarean section. Artificial ventilation was begun, and prostaglandin was administered. Saturation of percutaneous oxygen (SpO2) increased from 20% to 50%. Apgar scores were 2 and 2 at 1 and 5 minutes after birth, respectively. A chest radiograph showed severe cardiomegaly and a cardiothoracic ratio of 98% (Fig. 2). An echocardiography confirmed the prenatal diagnosis and showed massive tricuspid regurgitation. She continued to be hypoxic with SpO2 at 50% after intubation. Within 92 minutes after birth, surgery was initiated using cardiopulmonary bypass with an arterial cannula into the ascending aorta and bi-caval venous cannulae. The ductus arteriosus was ligated, and the tricuspid valve annulus was closed using a 0.4 mm expanded polytetrafluoroethylene patch with a 3 mm-diameter hole. The atrial septum and the dilated right atrial wall were excised widely. After aortic unclamping, the right ventricular wall was plicated longitudinally using 3-0 braids (Nespolene®, Alfresa, Osaka, Japan). An aorto-pulmonary shunt was constructed, using a 3.0 mm-diameter expanded polytetrafluoroethylene tube graft, between the brachiocephalic artery and the right pulmonary artery. During weaning from cardiopulmonary bypass, hypoxia and hypoventilation were observed. Therefore, HFV (fraction of inspired oxygen (FiO2), 100%) with inhaled nitric oxide (iNO) 20 ppm was used to successfully wean from cardiopulmonary bypass. The patient was returned to the intensive care unit with her chest open.
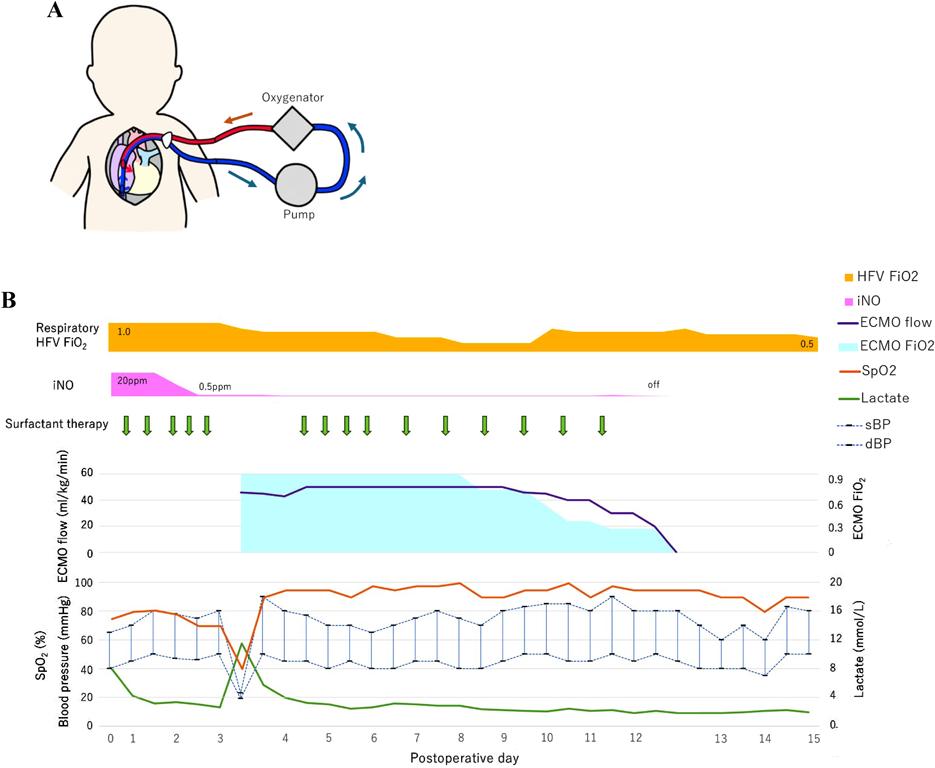
On the postoperative day 3, severe hypoxia suddenly developed (SpO2 decreased from 82% to less than 50%), followed by hypotension. An echocardiogram detected smooth flow across the aortopulmonary shunt and good left ventricular function. Therefore, we determined that the cause of hypoxia was due to pulmonary insufficiency, not because of an issue related to pulmonary blood flow. We decided to utilize VV ECMO. A 13 Fr double-lumen blood access catheter (Power Trialysis®, Becton Dickinson Inc., NJ, USA) was inserted from the right atrial appendage to the inferior cava vein. Venous blood was withdrawn from the tip of the catheter, and oxygenated blood was sent back into the atrium (Fig. 3A). VV ECMO (50 mL/kg/min, FiO2 100%) was initiated. Hemodynamics improved dramatically and stabilized. We performed systemic heparinization to maintain an Activated Clotting Time of 200 to 250 seconds during VV ECMO. HFV, minimal catecholamine support (dopamine: 2 µg/kg/min), and surfactant therapy were administered. We performed lung recruitment every day and reduced iNO from 20 to 1 ppm. After 7 days on ECMO, the lung fields on the chest radiograph improved and the ECMO flow was gradually decreased to the flow of 20 mL/kg/min with FiO2 30%. On postoperative day 12, VV ECMO was separated (Fig. 3B). The patient was weaned from iNO on the postoperative day 13 and had her chest closed on the postoperative day 24 with no problems in cardiac and lung function. She was discharged on postoperative day 106 (SpO2 90% in room air). The non-fenestrated Fontan circulation was established two years and nine months following the bidirectional Glenn procedure. Preoperative catheterization before the Fontan procedure showed pulmonary artery index of 215 mm2/m2 and pulmonary vascular resistance of 1.6 U·m2. The six-year-old has normal activity without a neurological deficit.
In Ebstein disease or TV dysplasia with severe tricuspid valve regurgitation, right ventricular exclusion improves neonatal mortality and morbidity.8) Right ventricular exclusion with/without right ventricular plication procedures can improve left ventricular function and provide expansion of the lungs. These thoracic structures are compressed by the dilated right atrium and the right ventricle. It was reported that the survival rates of the neonates undergoing the modified Starnes procedure were 87±2%, 87±2%, and 81±4% at 1, 5, and 10 years, respectively.9) Meanwhile, pulmonary hypoplasia and atelectasis due to cardiomegaly during the fetal stage may affect the postoperative condition. In fetal life, gross cardiomegaly with right atrial/ventricular dilatation occupying the major part of the thoracic cavity inhibits lung development, and this mechanism sounds similar to that in congenital diaphragm hernia. In congenital diaphragm hernia, the thoracic cavity is occupied by abdominal contents (the bowel, the stomach, the colon, the liver, and the kidneys), and growth of the lung is restricted at the early stage of fetal life (8–10 weeks of gestation). The cardiomegaly in TV dysplasia or Ebstein disease develops at the middle and the late stages of fetal life, and lung growth is restricted at the late stage of fetal life (the alveolar stage of lung development). Tanaka showed that the thickness of the medial wall of the intrapulmonary artery in tricuspid valve disease with gross cardiomegaly was less severe compared to that in diaphragm hernia.10) Therefore, in cases of TV dysplasia or Ebstein disease, even if the chest radiography shows the so-called wall-to-wall heart orientation immediately after birth, recovery of lung function can be still expected after surgery. It was deemed preferable for the baby to be delivered after 34 weeks, when lung maturity is typically achieved.11) In our patient, cardiomegaly developed after 33 weeks’ gestation; since no sign of fetal hydrops or increased pericardial fluid was observed, we decided on an earlier delivery within the full-term range. After surgery, the patient was effectively managed with HFV and surfactant therapy.
ECMO is an alternative and valid management for severe respiratory failure, although it is invasive. For congenital heart disease, veno-arterial (VA) ECMO is commonly used to support respiratory and cardiac function.12) However, in patients with single ventricular physiology and sufficient systemic ventricular function, VA ECMO may deliver oxygen insufficiently to the coronary arteries due to cardiac output interference, inducing secondary cardiac dysfunction. In contrast, VV ECMO supplies oxygenated blood to the coronary circulation, thereby improving cardiac function; in contrast, it cannot reduce ventricular preload to support ventricular function directly.12) In our patient, left ventricular function was sufficient enough to maintain systemic cardiac output, and atrial communication was opened at the Starnes procedure. Since the primary issue was respiratory failure rather than cardiac dysfunction, VV ECMO was determined to be the most appropriate choice. In addition to VV ECMO, HFV respiratory management, surfactant therapy, and lung recruitment were utilized to improve oxygenation and respiratory function. The patient was managed with her chest open, and the arterial and the right atrial purse string stitches were left reusable with adjusting tourniquets on since the surgical procedure. This setup allowed for prompt conversion to VA ECMO if the patient’s condition deteriorated. VA ECMO would have been considered if there were significant concerns about the patient’s cardiac function, such as low cardiac output or hemodynamic instability. While VA ECMO provides both cardiac and respiratory support, it carries a higher risk of complications including increased afterload on the heart. Ultimately, VV ECMO was selected as it was more suitable for this patient’s condition; that is, respiratory failure having been the main issue. The preparation for a possible switch to VA ECMO ensured that we could quickly respond to any changes in the patient’s clinical status. After chest radiography and hemodynamic improvement, weaning was initiated on day 7 of VV ECMO. Hemodynamic stabilization and chest radiographic findings were useful indicators for VV ECMO weaning.