Cardiac implantable electronic devices (CIEDs) are used to treat bradyarrhythmia, lethal arrhythmias such as ventricular fibrillation, and cardiac resynchronization therapy for heart failure.1) In adults, CIEDs are implanted via a transvenous endocardial approach and are often implanted in the subclavian region. In contrast, such devices are often implanted in the abdomen via a transthoracic epicardial approach in young children and patients with congenital heart disease with limited venous access to the heart.1) The number of abdominal CIEDs in Japan has not been reported, and the actual number is unknown.
Recently, frequent activation of the magnet response has been reported in pediatric patients with abdominal CIEDs due to electromagnetic interference (EMI) caused by a tablet internal speaker.2, 3) Magnet response function is intended to be used in operating rooms, in order to prevent synchronous pacing with radiofrequency or inappropriate shocks to patients by implantable cardiac defibrillators (ICDs) evoked by surgical equipment. In the presence of EMI, magnet response occurs in pacemakers; the devices are switched to an asynchronous pacing mode without synchronizing or inhibiting the patient’s own heartbeat, resulting in suspension of tachycardia detection and therapy for ICDs. If an unexpected magnet response occurred in a patient with a pacemaker implanted, asynchronous pacing would compete with patient’s own heartbeats, potentially causing discomfort to the patient and exhausting the battery. Furthermore, pacing during the T wave phase of the patient’s own heartbeat can induce fatal arrhythmias. Additionally, in patients with implanted CIEDs with defibrillators, there is a risk that appropriate treatment may not be given in the event of a fatal arrhythmia, eventually leading to death. Thus, for medical safety reasons, precautions should be taken to avoid issues with magnet response in patients with implanted CIEDs.4–10)
We conducted a questionnaire survey to determine the number of abdominal CIEDs, and to clarify how frequently incidental magnet response occurs due to tablet EMI nationwide in Japan.
From September to November 2024, we conducted a questionnaire survey among members of three societies (councilors of the Japanese Society of Pediatric Cardiology and Cardiac Surgery, members of the Japanese Society of Pediatric Electrocardiology, and members of the Japanese Society of Adult Congenital Heart Disease) dealing with pediatric cardiac patients or those (including adults) with congenital heart diseases. These are major disease groups of patients having abdominal CIED.
The survey questionnaire included the following five items: name of the institution and the department, name of the respondent, total number of abdominal CIEDs managed at the institution (regardless of age), whether the respondent had experienced any case of magnet response by a tablet, and finally the circumstances of the activation.
The responses to the questionnaire above were analyzed based on the following aspects: 1) the number of institutions managing abdominal CIEDs, distribution of types of the facilities (university hospital, general hospital, or children’s hospital), and the number of managed patients in each region of Japan (Hokkaido, Tohoku, Kanto, Chubu, Kinki, Chugoku, Shikoku, and Kyushu); 2) distribution of the number of patients managed at each institution; 3) the number of institutions reporting magnet response due to EMI and the number of patients who experienced the event; 4) the detail of magnet response due to EMI by a tablet (location, cause, and other circumstances).
The Ethics Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery approved this study (approval number; JSPCCS IRB 2024 No. 2).
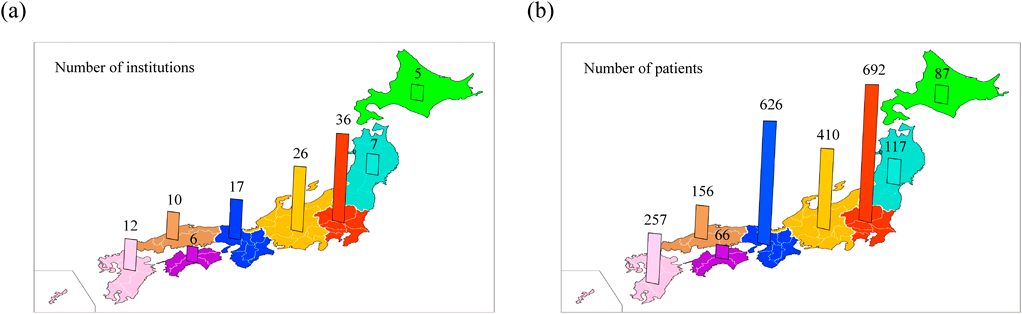
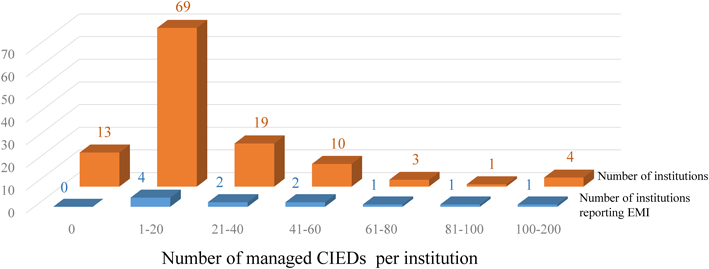
Survey responses were received from 119 institutions throughout Japan. These include 60 university hospitals (50%), 40 general hospitals (34%) and 19 children’s hospitals (16%). The total number of abdominal CIEDs was 2411. The cases were managed in institutions in Kanto, Chubu, Kinki, Kyushu, Chugoku, Tohoku, Hokkaido, and Shikoku regions (Fig. 1a). The number of cases managed in each region was in the following order: Kanto, Kinki, Chubu, Kyushu, Chugoku, Tohoku, Hokkaido, and Shikoku (Fig. 1b). The number of patients managed at each institution ranged from 0 (at 13 institutions) to 100–200 (at 4 institutions), with 1–20 being the most frequent category seen at 69 institutions (Fig. 2).
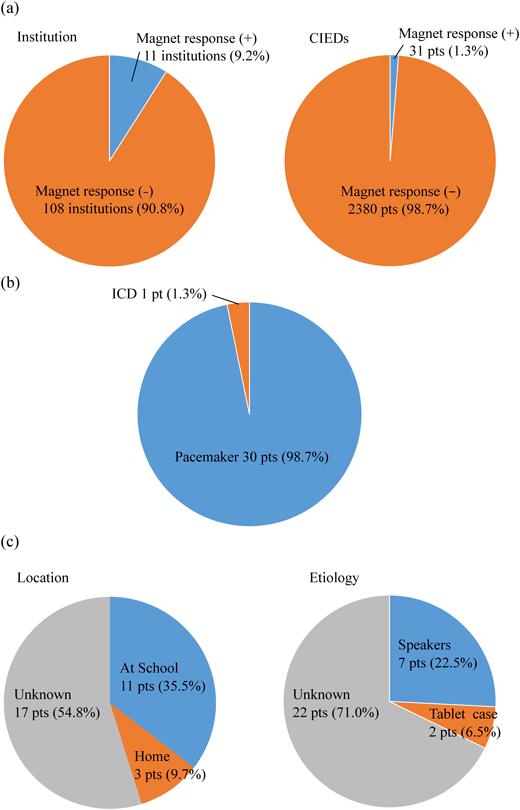
The magnet response due to EMI cause by a tablet was reported in 11 institutions (9.2%) counting 31 patients (1.3%) (Fig. 3a). In terms of the types of CIEDs, magnet response was activated in a pacemaker in 30 patients (98.7%) and an ICD in one patient (1.3%) (Fig. 3b).
The locations where magnet response occurred were at school, home, and unknown in 11 (35.5%), 3 (9.7%), and 17 patients (54.8%), respectively. The causes of these events were identified as laptop internal speakers in 7 (22.5%) and tablet magnets in 2 patients (6.5%) (Fig. 3c). Details of the EMI reports from each institution are shown in Supplementary table. In 7 patients with event situations realized, magnet response occurred when a tablet was placed close to the device. In another patient, magnet response was recorded when a computer was turned on during computer club at school. In the remaining one patient, magnet response occurred when the patient leaned over a stationary speaker. Because all these patients with a pacemaker remained asymptomatic, the occurrence of EMI was detected afterwards during routine pacemaker check-up. As for the patient with a trans-thoracic ICD, the device gave an alert with a beeping sound when an iPad was in use. Adverse events were not reported, such as induction of ventricular arrhythmia due to forced pacing or suppression of ICD therapy.
Our survey showed that the current number of abdominal CIEDs in Japan was 2411. This is the first report mentioning the total number of abdominal CIEDs in Japan. Regional differences were found in the number of cases, with the Kanto, Kansai, and Chubu regions having greater numbers of patients reflecting their populations. Of the 119 institutions, 101 (85%) managed 40 patients or less with abdominal CIEDs at each institution.
Thirty-one of the 2411 patients with abdominal CIEDs (1.3%) exhibited magnet response due to EMI induced by tablets. It was good to learn that all patients were asymptomatic and had no adverse events, such as induction of ventricular arrhythmias or suppression of ICD therapy. We suspect, nonetheless, that this result underestimates the actual incidence due to reporting biases. These biases include the absence of recording of magnet mode response in certain CIEDs and the requirement for specific configurations to retrieve magnet mode response records.11) This limitation, which leads to inter-institutional differences in CIED settings, also explains why the number of institutions reporting magnet response did not correlate with the number of CIEDs managed per institution.
CIEDs are affected by EMI, interference from strong magnetic fields including static magnetic fields, RF interference, and time-varying magnetic fields. The magnet response is typically triggered by static magnetic fields. In our study, we consider there are two possible causes of tablet-induced magnet response: permanent magnets in internal speakers and magnets in tablet frames or cases. Our study showed that permanent magnets in an internal speaker could have been the cause of the event in 7 patients, which were probably similar in those reported by Ota et al.2) These 7 patients were schoolchildren, experiencing an average of 39±91 episodes/month with a maximum of 167 episodes/month. Magnetic response was activated more frequently during school hours; which may have been caused by an educational tablet when the device was in contact with the abdomen of the children affected. In another 2 patients, magnet response was caused by magnets in a tablet case. Previous reports described similar instances of magnet response caused by magnets in the frame or cover of the iPad, or by using a device, such as a laptop, in contact with the chest while lying in bed.10, 12) Additionally, in our present report, magnet response was activated as soon as a computer was turned on in one patient; this phenomenon was possibly caused by the hard disk. Tiikkaja et al. reported an evidence of EMI noted by a warning sound when a laptop was turned on in an ICD patient; this event was attributed to EMI induced by the hard disk.12) They suspected that hard disks drive contained relatively strong permanent magnets and this caused EMI for the ICD.12) In our study, we were unable to identify the tablet parts that caused magnet response in the remaining 21 patients. We speculate, however, that the causes of magnet response could have been the magnets in tablet cases, permanent magnets in speakers built in tablets and laptops, or the electromagnetic field when a hard drive is turned on. Magnet response of abdominal CIEDs is most likely due to static magnetic fields caused by the close distance of magnets embedded in tablet devices.
The minimum magnetic flus density at which magnetic response is activated is 10 Gauss.5) iPhones and Apple watches have been extensively studied, and these were found to be unaffecting at a distance of only 3 cm.4–8) Tablet EMI can be prevented by holding a tablet a few centimeters away from the CIEDs, although the evidence for this is case-based studies.4–10) Conversely, tablet manufacturers recommend a distance of 15 cm from the CIED implantation site.
The patients were lying down and using the tablet on their abdomen, sitting cross-legged with putting the tablet on their feet, and so on, when the abdominal CIED and the tablet were in a close distance. Notably, this condition occurred only in patients with abdominal CIEDs. EMI due to tablets is considered rare in adult patients with thoracic CIEDs and has not received much attention. Most abdominal CIED implantation is indicated in young children with epicardial leads or in adults with congenital heart disease in whom venous access is limited. Pediatric cardiologists and physicians treating adult patients with congenital heart disease should be aware of the risk of EMI posed by use of a tablet in these postures.
For health care providers, it is very important to educate schools and families about safe use of tablets in terms of CIEDs. Specifically, it is recommended that tablets is to be used away from the device and body surface, and that the isolation distance should first be determined by referring to safety information provided through user manual/website of each tablet, as each tablet has various features of the isolation distance. If the value was not clearly stated, a 15 cm distance should be applied.11) If EMI occurs frequently, the priority is to find the cause, and to correct the circumstance. When the cause is not identified, it would not be unreasonable to consider turning magnet response function off after evaluating the benefits (preventing disruptions) and the risks (losing emergency magnet response action) by suspending the function of the CIEDs.
We were unable to determine the exact questionnaire response rate for this study, because the questionnaire was distributed to the members of the three particular societies. Although we collected questionnaires replies from all major institutions in Japan, we could not determine which institutions had not responded to the questionnaires or had not been involved in the survey. The questionnaires were not administered to institutions that are not affiliated with the three societies. Nevertheless, 106 out of 161 specialist training institutions nationwide for pediatric cardiology or adult congenital heart disease responded to the survey, resulting in the questionnaire response rate of 66%.
The number of patients with abdominal CIEDs obtained here does not represent a complete national count, as it was based on the questionnaire distributed to certain institutions.
The reported figure 1.3% for recorded magnet response counts only confirmed incidents. Due to the aforementioned reporting biases, EMI was unlikely detected in a certain proportion of patients, leading to underestimation of actual EMI occurrences.
The number of patients with abdominal CIEDs in Japan was 2411. Of these, magnet response due to EMI from tablets and laptops occurred in 1.3%. This figure most likely underestimate the actual incidence because of various limitations of this study. Patients with abdominal CIEDs, their families, and healthcare professionals should be reminded not to use tablet cases with magnets, tablets, or laptops close to the device.
謝辞Acknowledgments
We would like to express our deepest gratitude to the doctors in the institutions who participated in the study.
Conflicts of Interest
The authors declare no competing interests.
Ethics Approval and Consent to Participate
The Ethics Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery approved this study (approval number; JSPCCS IRB 2024 No. 2).
Author Contribution
Shuhei Fujita wrote the paper as the first author. Aya Miyazaki prepared the research plan, and Shuhei Fujita, Gaku Izumi, Tsugutoshi Suzuki, and Aya Miyazaki tabulated the contents of the questionnaire. Yasunobu Hayabuchi and Mari Iwamoto provided guidance in writing the paper and final proofreading, and all co-authors contributed to proofreading the paper.
Note
Supplementary table: Details of reports from each institution regarding magnet response due to EMI.
引用文献References
1) Nogami A, Kurita T, Abe H, et al: JCS/JHRS Joint Working Group: JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. J Arrhythm 2021; 37: 709–870
2) Ota A, Yamasaki H, Ogawa Y, et al: Effect of tablet personal computers on electromagnet interference in pediatric patients with implanted pacemakers in abdomen. Shindenzu 2024; 44: 180–188
3) Miyazaki A: Electromagnetic Interference to abdominal cardiac implantable electronic devices by tablet personal computers. Jpn J Electrocardiol 2024; 44: 189–190
4) Seidman SJ, Guag J, Beard B, et al: Static magnetic field measurements of smart phones and watches and applicability to triggering magnet modes in implantable pacemakers and implantable cardioverter-defibrillators. Heart Rhythm 2021; 18: 1741–1744
5) Féry C, Desombre A, Quirin T, et al: Magnetic field measurements of portable electronic devices: The risk inside pockets for patients with cardiovascular implantable devices. Circ Arrhythm Electrophysiol 2022; 15: e010646
6) Quirin T, Féry C, Nicolas H, et al: Quantification of the safety distance between ICDs and phones equipped with magnets. JACC Clin Electrophysiol 2021; 7: 1066–1068
7) Nadeem F, Nunez Garcia A, Thach Tran C, et al: Magnetic interference on cardiac implantable electronic devices from Apple iPhone MagSafe technology. J Am Heart Assoc 2021; 10: e020818
8) Greenberg JC, Altawil MR, Singh G: Letter to the Editor: Lifesaving therapy inhibition by phones containing magnets. Heart Rhythm 2021; 18: 1040–1041
9) Niyama D, Takesawa S, Tobata H: Effects of static magnetic fields on the cardiac implantable devices and their comparison. Jpn J Med Instrum 2023; 93: 26–32
10) Kozik TM, Chien G, Connolly TF, et al: iPad2(R) use in patients with implantable cardioverter defibrillators causes electromagnetic interference: The EMIT Study. J Am Heart Assoc 2014; 3: e000746
11) JHRS: Advisory on Magnet Responses Triggered by Tablets (February 10, 2025) https://new.jhrs.or.jp/blog/2025/02/10/wn20250210/
12) Tiikkaja M, Aro A, Alanko T, et al: Inappropriate implantable cardioverter-defibrillator magnet-mode switch induced by a laptop computer. Pacing Clin Electrophysiol 2012; 35: e177–e178