Respiratory syncytial virus (RSV) is an important cause of respiratory tract infections (RTIs) in infants and young children. Serious lower RTI is among the most common diseases that necessitate hospitalization in this age group1, 2) and is especially severe in those with risk factors such as preterm birth, chronic lung disease,3, 4) and congenital heart disease (CHD). When infants and young children with CHD contract RSV infection, the resulting disease can often become severe, possibly leading to the postponement or cancellation of the surgical treatment for CHD. Postoperative lung damage might persist, which could result in a prolonged respiratory disorder.5–12) Therefore, preventing and minimizing RSV infection are important for the management of infants and young children with CHD.
In Japan, the use of palivizumab, an anti-RSV monoclonal antibody, in infants and young children with a history of preterm birth or chronic lung disease was officially approved by health insurance in April 2002, and the guideline for its use was published.13) Meanwhile, in Western countries, the use of palivizumab for children with CHD was approved for the prevention of serious RTIs caused by RSV in infants and young children with hemodynamically significant CHD after the 2003 RSV season.14, 15) After that, a clinical study on children with CHD in Japan showed that the efficacy and safety of palivizumab were similar to those obtained in foreign studies.
Considering these circumstances and the results of a clinical trial conducted in Western countries16) and surveys in Japan,17, 18) to prevent and minimize the severity of RSV infection in infants and young children with CHD, the Japanese Society of Pediatric Cardiology and Cardiac Surgery published guideline for the use of palivizumab in infants and children with CHD in 200519) with the objective of defining the appropriate use of palivizumab by pediatric cardiologists, neonatologists, and pediatricians.
In Japan, the use of palivizumab for infants and young children with CHD was officially approved in October 2005; since then, severe RSV infections in infants and young children with CHD have been effectively prevented. Ten years later, the contents of the guideline were revised to fit the recent trends on the basis of past experiences and survey results.20) Please refer to the end of the document for an outline of the steps followed in the preparation of the 2019 guideline and the important points on its use.
Infants and young children with CHD, who are at a high risk of contracting RSV infection, are defined below. The administration of palivizumab to prevent and minimize the incidence of RSV infection is recommended.
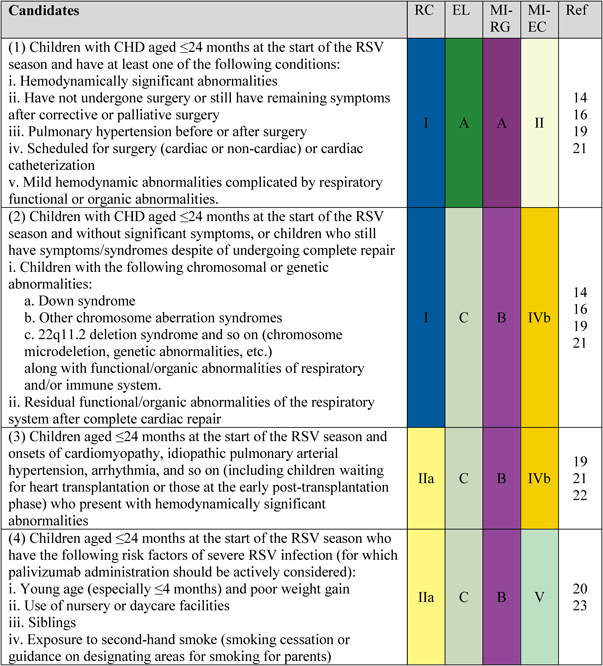
1. Candidates
Candidates are shown in Fig. 1.
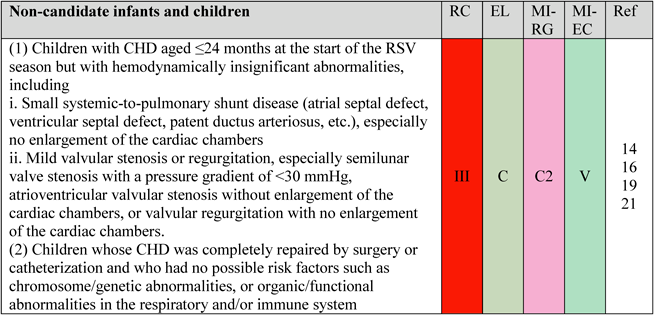
2. Non-Candidates
Palivizumab is not indicated for infants and children with certain conditions even if they have CHD and are ≤24 months of age at the beginning of the RSV season (Fig. 2). The heavy burden on patients and families due to the excessive administration of palivizumab to low-risk patients with mild conditions must be avoided.
1. Month of First Palivizumab Injection and Treatment Duration
To achieve high efficacy of palivizumab, the serum antibody titer needs to be at the level necessary for prevention prior to the start of the RSV season.
The timing of RSV outbreaks change every year depending on climate conditions and other factors and vary among regions in Japan.24) To develop a dosing plan, a method was devised to estimate the start of the RSV season on the basis of the Epidemiological Surveillance of Infectious Diseases from each prefecture for the last several years and the number of patients at sentinel sites.25–27) (RSV infection was designated as a sentinel site reporting Class V Infectious Disease by the 2003 Amendment to the Infectious Diseases Control Law in Japan.) According to this method and the trend of the annual incidence of RSV infection, unifying the month of first administration for each year and each prefecture is ideal. Regarding the end of the season, each prefecture and year demonstrate different patterns; thus, a clear standard to determine the end of the season is difficult to develop. Similar to the start of the season, the month in which the season ends should be estimated on the basis of data from the Epidemiological Surveillance of Infectious Diseases in each prefecture for the last several years. Repeated administration of palivizumab resulted in a sufficient elevation in serum antibody titer, and its efficacy could be maintained for a month post administration. Post-marketing survey results showed that the number of administrations ranged from 1 to 9, but no report has indicated the association of adverse events with the number of administrations.28, 29)
As RSV outbreaks change year after year, pediatricians involved with perinatal care and palivizumab administration in each prefecture should lead deliberations and determine the administration start month, administration duration, and number of administrations. It is useful to share information with processors from the Health Insurance Claims Review and Reimbursement Services and National Health Insurance Organization.30)
When administering palivizumab to infants or young children discharged from the neonatal intensive care unit/growing care unit, considering the time necessary to increase the serum palivizumab concentration, it is recommended that the dose is given at least 3 days prior to discharge.
The maintenance period of an effective palivizumab concentration is shorter after the first administration than after the second dose; thus, a shorter interval from the first administration is recommended for the post-discharge administration.
2. Administration of Palivizumab to Children after Surgical Procedures Involving Cardiopulmonary Bypass
Serum palivizumab concentration has been reported to decrease significantly after surgery using cardiopulmonary bypass (Fig. 3).14, 16)
3. Dose and Dosing Schedule
The palivizumab dose and dosing schedule are shown in Fig. 4.
4. Notice for the Intramuscular Injection of Palivizumab:
- ①It is to be given intramuscularly only, and intravenous administration should be avoided.
- ②It should not be mixed with other injectable agents.
- ③It should be injected in the muscle, ideally in the anterolateral aspect of the thigh, and the gluteal muscle should not be used as an injection site because of the risk of injury to the sciatic nerve.
- ④Areas in which nerves run should not be used as injection sites.
- ⑤Repeated injections in the same area should be avoided.
- ⑥When severe pain or backflow of blood into the syringe is observed during injection, the needle should be withdrawn immediately, and the injection site should be changed.
Please refer to Intramuscular Inoculation of Vaccines for Children (Revised Version) by the Japan Pediatric Society for standard intramuscular injection methods.31)
1. Precautions for Palivizumab Injection32):
- 1) When patients have RSV infection while receiving palivizumab injections, continuous administrations are recommended during the RSV season to prevent severe lower RTI due to reinfection.
- 2) In children with a bleeding tendency due to thrombocytopenia or other coagulation disorders, serious conditions could develop; thus, it is recommended that palivizumab should be carefully administered with the application of pressure on the injection site until hemostasis is confirmed.
- 3) Muscular contracture reportedly develops from intramuscular injection of antibiotics; thus, palivizumab should be administered with utmost care.
- 4) In children with moderate to severe acute infection or febrile disease, or an unstable circulatory and/or respiratory system, palivizumab administration should be postponed unless its benefit outweighs its risks. Mild febrile diseases such as upper RTIs are usually not reasons to postpone palivizumab administration.
- 5) Whether palivizumab is effective for treating established RSV infections has not been clarified.
2. Side Effects
Note the following two major side effects32):
- 1) Anaphylactic shock (unknown frequency) — Observe the patient carefully and stop the administration if cyanosis, cold sweats, blood pressure decrease, breathing difficulties, stridor/wheezing, or tachycardia is observed, and provide appropriate treatment such as epinephrine therapy (1 : 1000).
- 2) Thrombocytopenia (unknown frequency)—Observe the patient carefully, and if any abnormalities are observed, provide the appropriate treatment such as discontinuation of the palivizumab administration.
3. Interactions with Underlying Diseases, Other Drugs, and so on
- 1) No adverse events associated with palivizumab injections have been reported in infants or young children with the following conditions:
- i) food allergy;
- ii) treatment with immunoglobulin preparations (for Kawasaki disease, etc.);
- iii) open-heart surgery;
- iv) palivizumab therapy during the previous RSV season; and
- v) a history of RSV infection.
- 2) No reports have indicated adverse drug reactions resulting from interactions between palivizumab and other drugs in clinical trials in Japan or foreign countries.
- 3) In foreign clinical trials in which palivizumab was combined with inactivated or live vaccines, the number of adverse events did not increase. Increased adverse events have not been reported in Japan.33) As palivizumab binds specifically to RSV, palivizumab injections are believed not to prevent immune response to vaccines. Therefore, the vaccination schedule need not be changed during palivizumab administrations.
Importance of Basic Infection Control
Basic infection control is essential even when palivizumab injections are administered. Educating parents about infection control is important because the management of high-risk children requires their cooperation. Parents should be educated not only about RSV infection but also about the basic precautions needed to prevent RTIs. It is also recommended that parents are well educated to strictly adhere to the administration schedule to maintain the efficacy of palivizumab.
Basic information to prevent RSV infections includes1, 2)
- 1) Source of infection: infants with RSV infection, family members or medical staffs, and so on who are infected with RSV during the season;
- 2) Infection pathway: mainly the nasal mucosa and conjunctiva;
- 3) Mode of transmission: contagious infection (through hands contacting secretions from infected individuals) and droplet infection (through relatively large droplets such as saliva and sputum at a distance of <1 m); and
- 4) Preventive methods: standard precaution along with the prevention of contagious and droplet infections by avoiding crowded spaces and monitoring for cold symptoms in parents and siblings.
RSV Infection Countermeasures at Medical Facilities
RSV can lead to serious symptoms in high-risk children by spreading within medical facilities. Therefore, children with RSV infection should be isolated to prevent the infection of high-risk children hospitalized at the facility. When an RSV outbreak is confirmed, appropriate infection countermeasures are implemented at facilities and wards by an infection control team (Fig. 5).34, 35)
Important Steps for the Preparation and Use of This Guideline
The current scientific basis for implementing a systematic review is insufficient. Upon discussing this issue with related academic societies, the consensus guidelines were summarized on the basis of a consensus that reflects the latest evidence and medical conditions.
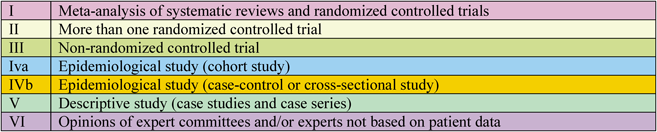
This revised guideline also includes the recommended class and evidence level determined by the authors on the basis of domestically and internationally published papers, and ultimately chosen by committee members and external evaluators. Similar to foreign guidelines that follow traditional guidelines, the recommended class (RC) and evidence level (EL) are indicated (Figs. 6 and 7). Moreover, Medical Information Network Distribution Service (MINDS)-recommended grades (MI-RG) and MINDS-evidence classification (MI-EC) were applied from the “2007 MINDS Handbook for Clinical Practice Guideline Development,”36) issued by the Japan Council for Quality Health Care (Figs. 8 and 9). The description of the evidence level in the Western guidelines was based on the idea that randomized clinical trials feature a higher evidence level than registered studies. By contrast, the evidence classification of MINDS shows the types of trials and studies that are the basis of the evidence, although these descriptions differ.
Recommendations made in the treatment guideline should not be forced but rather considered as a reference for treatment options. Patients and medical providers should be given discretion to choose the optimum treatment through their cooperation.37) Therefore, the consensus guidelines also list diseases and/or conditions that are not officially approved.
The recommended grade is comprehensively determined on the basis of the evidence level and number, variations in the conclusion, clinical efficacy, clinical applicability, and evidence about adverse events and cost-benefits.
謝辞Acknowledgments
This review article (secondary publication) was supported by a research grant from the Japanese Ministry of Health, Labor, and Welfare (H30-intractable diseases-general-010). We would like to thank Editage (www.editage.com) for English language editing.
Originally published in Pediatric Cardiology and Cardiac Surgery, Vol. 35 (2019), S2.1–S2.7
引用文献References
1) Hall CB, Weinberg GA, Iwane MK, et al: The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360: 588–598
2) Nair H, Nokes DJ, Gessner BD, et al: Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010; 375: 1545–1555
3) American Academy of Pediatrics Committee on Infection Diseases and Committee on Fetus and Newborn: Prevention of respiratory syncytial virus infections: Indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics 1998; 102: 1211–1216
4) Xavier CE, Giuffre L, Kimpen JLL, et al: Guideline for the use of Synagis (palivizumab), a humanized monoclonal antibody, for the prevention of respiratory syncytial virus (RSV) disease in high risk infants A consensus opinion. Infect Med 1999; 16: 29–33
5) MacDonald NE, Hall CB, Suffin SC, et al: Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med 1982; 307: 397–400
6) Fixler DE: Respiratory syncytial virus infection in children with congenital heart disease: A review. Pediatr Cardiol 1996; 17: 163–168
7) Navas L, Wang E, de Carvalho V, et al: Pediatric Investigators Collaborative Network on Infections in Canada: Pediatric Investigators Collaborative Network on Infections in Canada: Improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. Pediatric Investigators Collaborative Network on Infections in Canada. J Pediatr 1992; 121: 348–354
8) Tsuda T, Sawada Y, Ikeda K, et al: RSV infection of left-to-right shunt CHD with pulmonary hypertension. J Jap Pediatr Soc 1982; 86: 2076–2082
9) Tajima G, Ishii S, Sasabe M, et al: Clinical image of pediatric RSV infection at our department. J Hirosh Med Assoc 1999; 52: 22–28 (in Japanese)
10) Saijo M, Ishii T, Kokubo M, et al: Respiratory syncytial virus infection in lower respiratory tract and asthma attack in hospitalized children in North Hokkaido, Japan. Acta Paediatr Jpn 1993; 35: 233–237
11) Saijo M, Takahashi S, Kokubo M, et al: The role of respiratory syncytial virus in acute bronchiolitis in small children in northern Japan. Acta Paediatr Jpn 1994; 36: 371–374
12) Saijo M, Takimoto M, Takahashi Y: Epidemiological study on respiratory syncytial virus lower respiratory tract infections in Northern Hokkaido, Japan. Jap Assoc Infect Dis 1994; 68: 1–6 (in Japanese)
13) Nishida H, Fujimura M, Takeuchi Y, et al: Prevention of RSV infection (Guidelines for the Use of Palivizumab in Japan). J Jap Pediatr Soc 2002; 106: 1288–1292 (in Japanese)
14) Tulloh R, Marsh M, Blackburn M, et al: Working Group of the British Paediatric Cardiac Association: Working Group of the British Paediatric Cardiac Association: Recommendations for the use of palivizumab as prophylaxis against respiratory syncytial virus in infants with congenital cardiac disease. Cardiol Young 2003; 13: 420–423
15) American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn: Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003; 112: 1442–1446
16) Feltes TF, Cabalka AK, Meissner HC, et al: Cardiac Synagis Study Group: Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143: 532–540
17) Saji T, Nakazawa M, Harada K: (Japanese Society of Pediatric Cardiology and Cardiac Surgery academic committee): Survey on the efficacy and safety of an anti-RSV monoclonal antibody, palivizumab, on infants and young children with congenital heart disease. Pediatr Cardiol Cardiac Surg 2004; 20: 45–49 (in Japanese)
18) Saji T: Evidence Evaluation to Expand Indications of Drugs for Infants and Young Children with Congenital Heart Disease 1. Retrospective Survey on Infection Preventative Effect of Anti-RSV Antibody for Children with Heart Diseases. Health Labour Sciences Research Grant (H15-risk-004) Report of Joint Research in 2003 on the Drugs and Medical Techniques Risk Evaluation Project, 2003, pp 78–92 (principal investigator: Onishi S) (in Japanese)
19) Japanese Society of Pediatric Cardiology and Cardiac Surgery Guideline Development Committee: (chairman: Nakazawa M, members: Saji T, Fukiko Ichida, and Oyama K, external evaluator: Kusuda S, evaluator: Harada K): Guidelines for the use of palivizumab in infants and young children with congenital heart disease. Pediatr Cardiol Cardiac Surg 2005; 21: 60–62 (in Japanese)
20) Andabaka T, Nickerson JW, Rojas-Reyes MX, et al: Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013; 4: CD006602
21) Tulloh RMR, Medrano-Lopez C, Checchia PA, et al: CHD and respiratory syncytial virus: Global expert exchange recommendations. Cardiol Young 2017; 27: 1504–1521
22) Bollani L, Baraldi E, Chirico G, et al: Italian Society of Neonatology: Italian Society of Neonatology: Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV). Ital J Pediatr 2015; 41: 97
23) Lanari M, Prinelli F, Adorni F, et al: Study Group of Italian Society of Neonatology on Risk Factors for RSV Hospitalization: Study Group of Italian Society of Neonatology on risk factors for RSV hospitalization: Risk factors for bronchiolitis hospitalization during the first year of life in a multicenter Italian birth cohort. Ital J Pediatr 2015; 41: 40
24) Shobugawa Y: RSV infection: Climatic condition for summer season. Paediatr Jpn 2018; 59: 363–368 (in Japanese)
25) Kusuda S: Monoclonal antibody preparations. Perinat Med 2018; 48: 155–158 (in Japanese)
26) Yamagami H, Kimura H, Hashimoto T, et al: Detection of the onset of the epidemic period of respiratory syncytial virus infection in Japan. Front Public Health 2019; 7: 39
27) Kanou K, Arima Y, Kimura H, et al: Epidemiological study of RSV infections in Japan: Regional nature of RSV season based on the National Epidemiological Surveillance of Infectious Diseases. J Jap Assoc Infect Dis 2018; 92suppl: 499
28) Abbott Japan: Result of Post-marketing Surveillance for 50 mg or 100 mg Synagis® for intramuscular injection. October 2010. (in Japanese)
29) ClinicalTrials.gov: Synagis® Liquid 50 mg, 100 mg for Intramuscular Injection Special Investigation in immunocompromised Children With Synagis®.; NIH. U.S. National Library of Medicina. https://clinicaltrials.gov/ct2/show/study/NCT02016690 (as of February 3, 2019)
30) Arai J, Miyazono Y, Hidaka D, et al: Examination of the appropriate timing for palivizumab administration in Ibaraki Prefecture. J Jap Soc Neonat Health Dev 2018; 30: 331 (in Japanese)
31) Japan Pediatric Society: “Intramuscular Inoculation of Vaccines for Children (Revised)”. Japan Pediatric Society. https://www.jpeds.or.jp/uploads/files/20160708_kinnnikunaisesshu.pdf (as of February 3, 2019) (in Japanese)
32) Abbvie: Synagis. A-CONNECT. https://a-connect.abbvie.co.jp/-/media/assets/pdf/products/synagis/ syn_l.pdf (as of March 21, 2019) (in Japanese)
33) Toishi S, Ishiwada N, Osone Y, et al: Safety of simultaneous vaccination for children being administered with palivizumab. J Jap Pediatr Soc 2017; 121: 1063–1066 (in Japanese)
34) Silva Cde A, Dias L, Baltieri SR, et al: Respiratory syncytial virus outbreak in neonatal intensive care unit: impact of infection control measures plus palivizumab use. Antimicrob Resist Infect Control 2012; 1: 16
35) Hammoud MS, Al-Taiar A, Raina A, et al: Use of palivizumab with other infection control measures to control respiratory syncytial virus outbreak in neonatal care units. J Trop Pediatr 2016; 62: 409–414
36) MINDS Handbook for Clinical Practice Guideline Development Committee ed. Fukui T, Yoshida M, and Yamaguchi N, eds.: MINDS Handbook for Clinical Practice Guideline. Igaku-Shoin Ltd., 2007
37) Japan Council for Quality Health Care: EBM Medical Information Network Distribution Service (MINDS). http://minds.jcqhc.or.jp (as of February 3, 2019) (in Japanese)