Hypoplastic left heart syndrome (HLHS) and right atrial isomerism (RAI) are congenital heart diseases with particularly poor prognoses.1, 2) The circulatory dynamics of these diseases affect child development, starting in the fetal stage, and can be fatal if appropriate postnatal treatment is not provided.3, 4) Atrioventricular regurgitation (AVVR) is a factor that is strongly associated with the prognosis of these diseases. In cases of severe regurgitation, it is not uncommon for the atrioventricular valve to require surgical intervention in the neonatal stage.5–7).
In adults, the use of 3D echocardiography has advanced our understanding of the anatomy and functions of the tricuspid valve. Recently, analyses focusing on the tricuspid annulus have attracted attention. However, little research has been conducted on congenital heart diseases, especially on the tricuspid and common atrioventricular valves in the univentricular circulation,8–12) and methods for evaluating annulus function at the clinical level have not been established.
In this study, we performed X-plane tissue tracking using a transthoracic 3D probe to evaluate the function of the tricuspid annulus and analyzed changes in the area of the tricuspid annulus during the cardiac cycle.
Subjects
There were 32 patients in the HLHS and RAI group (15 HLHS, 17 RAI). Their median age was 3.0 years (0.1–18.1 years) and they included 13 boys. No distinction was made based on whether they had undergone cardiovascular surgery. There were 53 patients in the normal group. Their median age was 8.7 years (6.7–12.2 years) and they included 29 boys. The patients in the normal group were those who had no abnormal findings in echocardiography examinations for school-age children performed at Shizuoka Children’s Hospital in 2013–2014.
Methods
Equipment: PHILIPS IE-33 (Philips Medical Systems, Best, The Netherlands), 3D matrix probe (X-7, X-5).
Recording
The transthoracic 3D matrix probe was used to depict an apical 4-chamber view. Next, the tricuspid or common atrioventricular annulus was depicted in the center of the echocardiography screen, without leaving out the margins, and used to record the X-plane (4-chamber view and orthogonal view). During recording, the patients were placed in a lateral recumbent position, appropriate for an apical cardiac view and asked to hold their breath as much as possible.
Analysis
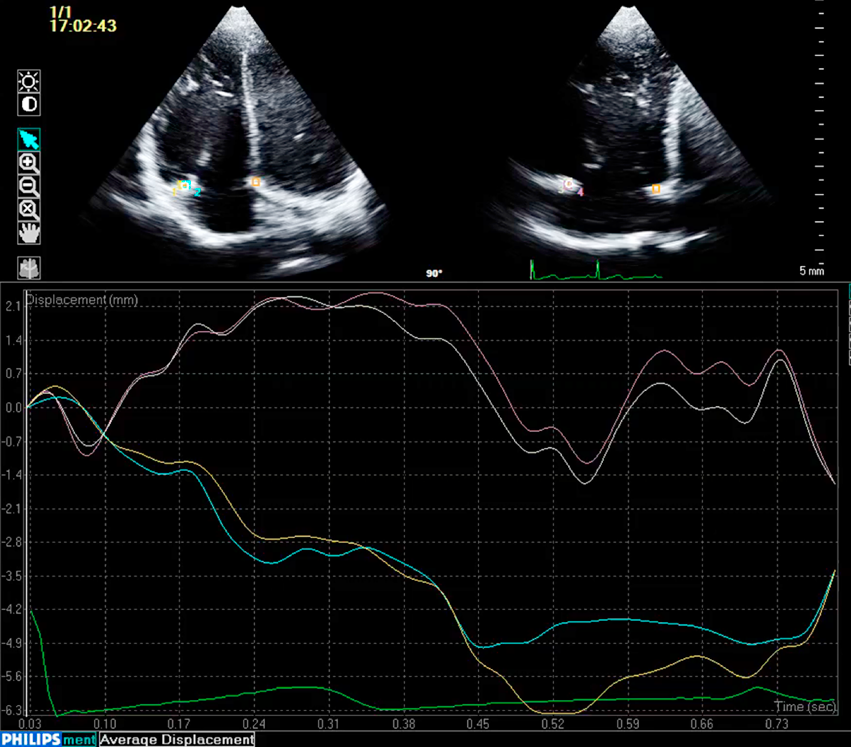
Tissue motion annular displacement, a function of QLAB 9.0 (Philips Medical Systems, Best, The Netherlands), was used to measure the diameter of the apical 4-chamber view in the septolateral direction (A) and the diameter orthogonal to this in the anteroposterior direction (B) by tracking the annulus during 1 cardiac cycle (Fig. 1). Assuming the annulus to be elliptical, we calculated its area using the 2 diameters with the following formula: annulus area=πAB/4. This was measured at each phase of the cardiac cycle to analyze the change in the tricuspid annulus area between phases. For the comparisons, we used values normalized to body surface area (annulus area / body surface area).
The changes in the tricuspid annulus and the common atrioventricular annulus area between phases were not uniform. They were classified into 3 types based on their pattern: decreased during systole (Type-1), increased during systole (Type-2), and no significant change (Type-3). For Type-3, no significant areal change was defined as a change of ≤10%.
Patients in the HLHS and RAI group were classified based on the grade of regurgitation into a low-grade AVVR group of cases with mild or lower regurgitation and a high-grade AVVR group of cases with moderate or higher regurgitation; comparisons were made between the groups. To investigate the relationship between the annulus movement pattern and valve function prognosis in the HLHS and RAI group, we examined changes in regurgitation after the analysis and whether valves underwent surgical interventions. In the prognosis analysis, the endpoint in the low-grade group was when regurgitation worsened to moderate or higher and the endpoint in the high-grade group was when a surgical intervention (valvuloplasty or valve replacement) was required.
The t-test and Fisher’s exact test were used to examine differences between the groups, and the log-rank test was used to examine the prognosis of valve function. A p<0.05 was considered statistically significant.
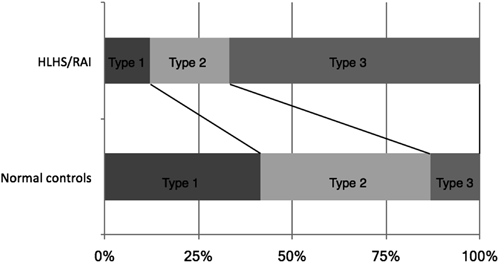
In the normal group, there were 22 (42%), 24 (45%), and 7(13%) cases of Type-1, Type-2, and Type-3, respectively, while in the HLHS and RAI group, there were 4 (13%), 7 (22%), and 21 (65%) cases of Type-1, Type-2, and Type-3, respectively. Type-3 cases were significantly more common in the HLHS and RAI group than in the normal group (p<0.01) (Fig. 2).
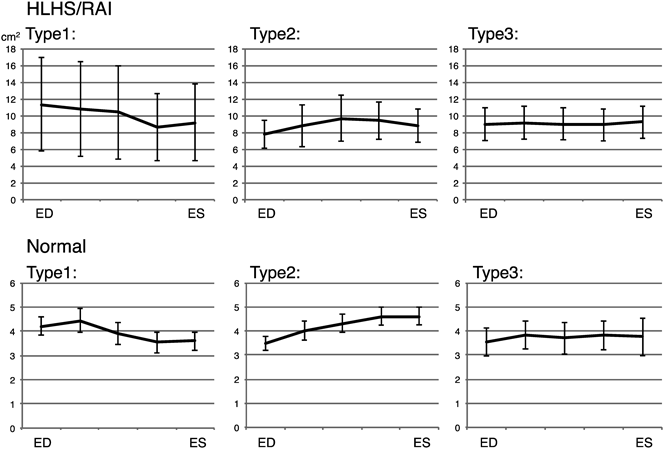
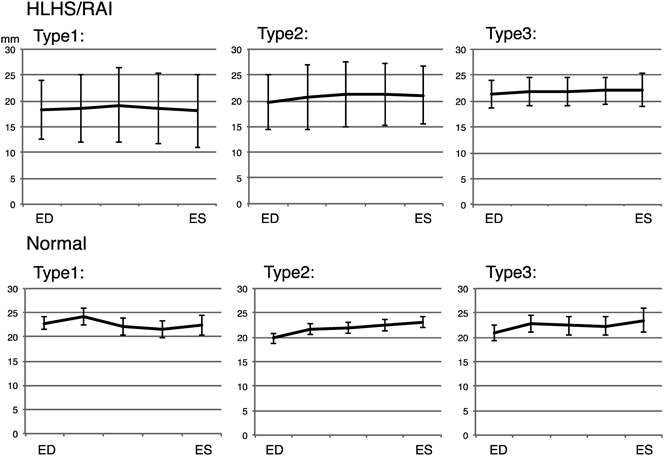
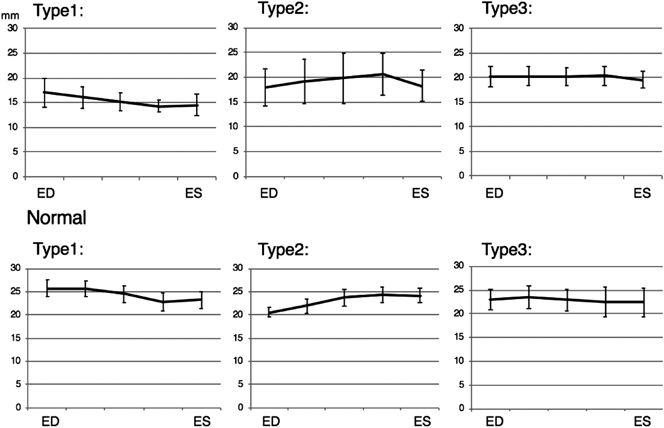
The maximum areal changes based on the end-diastolic annulus area were as follows: normal group: Type-1−24%, Type-2+31%, Type-3+9%; HLHS and RAI group: Type-1−25%, Type-2+28%, Type-3+2% (Fig. 3). The rates of change in septolateral diameter were as follows: normal group: Type-1−16%, Type-2+19%, Type-3+11%; HLHS and RAI group: Type-1+2%, Type-2+8%, Type-3+4% (Fig. 4). The rates of change in anteroposterior diameter were as follows: normal group: Type-1−15%, Type-2+19%, Type-3+4%; HLHS and RAI group: Type-1−19%, Type-2+16%, Type-3+1% (Fig. 5). The rate of septolateral diameter decrease during systole was smaller in the HLHS and RAI group, not only for Type-3 cases, but also for Type-1 and Type-2 cases.
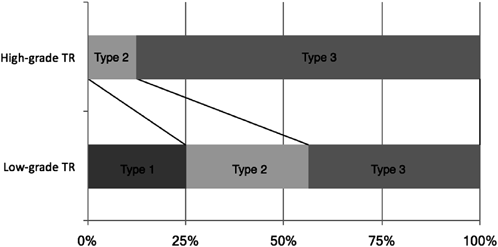
The low and high grade AVVR groups each consisted of 16 cases. The low-grade group had 4 (25%), 5 (31%), and 7 (43%) cases of Type-1, Type-2, and Type 3, respectively; the high-grade group had 0, 2 (12%), and 14 (88%) of Type-1, Type-2, and Type-3, respectively, showing Type-3 to be more common in the high-grade group (p<0.01) (Fig. 6). Table 1 shows a comparison of the tricuspid annulus areas for each type at end-diastole and end-systole in the normal, low-grade regurgitation, and high-grade regurgitation groups. The annulus areas were larger in the low-grade and high-grade AVVR groups than in the normal group. The annulus area did not differ significantly between the low- and high-grade groups (p=0.28).
Table 1 TV area comparison | Type 1 | Type 2 | Type 3 |
|---|
| ED | ES | ED | ES | ED | ES |
|---|
| Normal | 4.20 (±0.90) | 3.59 (±0.96) | 3.49 (±0.76) | 4.61 (±0.90) | 3.52 (±0.79) | 3.76 (±1.06) |
| Low-grade TR | 11.3 (±5.69) | 9.20 (±4.67) | 8.54 (±2.13) | 9.90 (±2.40) | 7.46 (±2.70) | 7.92 (±2.75) |
| High-grade TR | none | 6.20 (±0.57) | 6.75 (±0.35) | 9.79 (±5.10) | 9.90 (±5.30) |
| Unit: cm2, ED: end diastole, ES: end systole. |
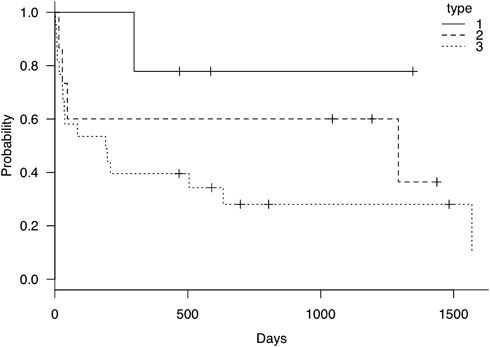
Fig. 7 shows the results of the analysis of the regurgitation prognosis in the HLHS and RAI group. Worsening regurgitation was observed in 6 patients (2 cases Type-2, 4 cases Type-3). Fifteen patients required surgical interventions, with most being Type-3 (1 case Type-1, 2 cases Type-2, 12 cases Type-3). The Kaplan–Meier method indicated that the regurgitation prognosis did not differ significantly between the types (p=0.23).
The tricuspid valve is a complex composed of an annulus and septal, anterior, and posterior leaflets, which are connected via chordae tendineae and papillary muscles. Each of these structural components moves together in close precision. The functions of the annulus are to maintain an appropriate diameter and area during the changes caused by myocardial expansion and contraction, and coordinate the movement of the leaflets, chordae tendineae, and papillary muscles to smoothly draw blood into the right ventricle and suppress backflow during ejection. In the present study, we analyzed the changes in the annulus area between phases of the cardiac cycle to evaluate the function of the tricuspid annulus.
We found that the area of the tricuspid annulus did not change uniformly; however, it could be classified into 3 types, based on the pattern of change. Studies using 3D echocardiography have found that the annulus area decreases during systole in normal tricuspid valves.13–15) To compensate for the coaptation of the septal leaflet, which has little mobility, the decrease in septolateral diameter during systole was considered to be particularly important to maintaining valve function.16) However, in our study, there were Type-2 and Type-3 cases in the normal group. Type-2, in particular, showed an increase in diameter in the septolateral direction during systole, which differs from the conventional understanding of tricuspid annulus function. Some cases of this type exhibited increased anteroposterior diameter as well. In this state, excess leaflet area appears to be necessary to suppress regurgitation.
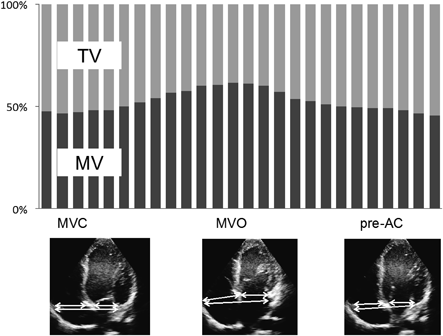
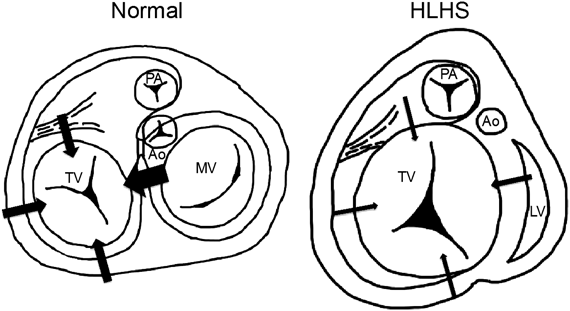
Interaction with the mitral valve is a factor that strongly influences changes in the tricuspid annulus area. In a normal heart, the septolateral diameter of the tricuspid annulus decreases during systole due to interaction with the mitral valve (Fig. 8). The diversity of changes in the annulus area are thought to arise from the degree of this interaction and differences in the change in the anteroposterior diameter. In the HLHS and RAI group, in contrast, this interaction is absent or weakened, resulting in less change in the tricuspid annulus area. In the present study, reduced rates of changes in septolateral diameter were observed, not only in Type-3 cases in the HLHS and RAI group, but also in Type-1 and Type-2 cases, suggesting a relationship between mitral valve interaction and tricuspid annulus function.
In the HLHS and RAI group, the annulus expanded to something close to a perfect circle (Fig. 9). This reduced the coaptation height between the leaflets, which worsened tricuspid regurgitation. Furthermore, volume loading from tricuspid regurgitation increases wall stress at the basal level, where the inner diameter is large. This decreases myocardial contraction at the basal level and further expands the tricuspid annulus, creating a negative spiral. The large number of Type-3 cases in the high-grade AVVR group and the high rates of worsening regurgitation and surgical interventions among Type-2 and Type-3 cases suggest a close relationship between reduced tricuspid annulus function and the severity of valvular regurgitation.
Analyses of tricuspid annulus function have been conducted with transesophageal 3D echocardiography, mainly in adults with right heart failure.13–15) However, performing transesophageal echocardiography on children requires general anesthesia, significantly hindering the application of this method, with even greater difficulties in infants. The above demonstrates the desirability of establishing a different evaluation method, but the challenge lies in creating something that is noninvasive, versatile, and has enough time resolution to be used with the rapid heart rates of children. Our method of analysis uses a transthoracic 3D probe and is noninvasive. Further, the median frame rate obtained with the transthoracic 3D probe in our study was 38 fps (24–65 fps), which is a sufficient degree of temporal resolution.
One advantage of this analysis method is that recordings are easy. The apical 4-chamber view needs to be depicted accurately and the tricuspid annulus needs to be kept within the depiction range, but this does not require any special skills. The feasibility of the analysis is affected by the range of tricuspid annulus depiction and image quality. In the present study, we obtained images that could be analyzed in 71% of the normal group (53/75 cases). As these recordings were made during health checks, in some cases, the tricuspid annulus was poorly depicted. We believe the analysis rate would be higher if recordings were focused on the tricuspid annulus. Another advantage is that the tracking data can be uploaded to a spreadsheet program where area calculations can be performed easily.
Our analysis method has some limitations. While we calculated the area using 2 orthogonal views of the tricuspid annulus, ideally the views should intersect at the center of the tricuspid annulus. In the analysis, we selected images depicting intersection points that were in the center of the annulus; however, there is no objective index for determining whether an intersection point is in the center of the annulus, and thus deviation of the intersection points may have affected the analysis. Further, while we assumed the annulus to be an ellipse on a single plane, in reality, changes in the tricuspid valve do not occur on the same plane, but in a 3D saddle shape. A difference between the assumed shape and the actual shape/change is a problem with this method. In addition, the age difference between the HLHS and RAI group and the normal group may also have influenced the results. To demonstrate the usefulness of this method more clearly, we would need to compare 3D echocardiography, CT, and MRI results in a more uniform population.17, 18)
The HLHS and RAI group exhibited reduced atrioventricular annulus function, which was part of the mechanism causing AVVR. Our method enables the evaluation of tricuspid annulus function in a simple, minimally invasive manner and may be useful for deepening our understanding of the pathology behind tricuspid regurgitation.
Conflicts of Interest
The authors have no conflicts of interest to disclose in relation to this paper.
Originally published in Pediatric Cardiology and Cardiac Surgery, Vol. 35 (2019), No. 1, pp. 30–37
引用文献References
1) Loomba RS, Nijhawan K, Anderson R: Impact of era, type of isomerism, and ventricular morphology on survival in heterotaxy: Implications for therapeutic management. World J Pediatr Congenit Heart Surg 2016; 7: 54–62
2) Yabrodi M, Mastropietro CW: Hypoplastic left heart syndrome: From comfort care to long-term survival. Pediatr Res 2017; 81: 142–149
3) Degenhardt K, Rychik J: Fetal situs, isomerism, heterotaxy syndrome: Diagnostic evaluation and implication for postnatal management. Curr Treat Options Cardiovasc Med 2016; 18: 77–87
4) Frommelt MA: Challenges and controversies in fetal diagnosis and treatment: Hypoplastic left heart syndrome. Clin Perinatol 2014; 41: 787–798
5) Feinstein JA, Benson DW, Dubin AM, et al: Hypoplastic left heart syndrome: Current considerations and expectations. J Am Coll Cardiol 2012; 59 Suppl: S1–S42
6) McGovern E, Kelleher E, Potts JE, et al: Predictors of poor outcome among children with heterotaxy syndrome: A retrospective review. Open Heart 2016; 3: e000328
7) Shamszad P, Gospin TA, Hong BJ, et al: Impact of preoperative risk factors on outcomes after Norwood palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2014; 147: 897–901
8) Bautista-Hernandez V, Brown DW, Loyola H, et al: Mechanisms of tricuspid regurgitation in patients with hypoplastic left heart syndrome undergoing tricuspid valvuloplasty. J Thorac Cardiovasc Surg 2014; 148: 832–838, discussion, 838–840
9) Bharucha T, Honjo O, Seller N, et al: Mechanisms of tricuspid valve regurgitation in hypoplastic left heart syndrome: A case-matched echocardiographic-surgical comparison study. Eur Heart J Cardiovasc Imaging 2013; 14: 135–141
10) Kutty S, Colen T, Thompson RB, et al: Tricuspid regurgitation in hypoplastic left heart syndrome: Mechanistic insights from 3-dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging 2014; 7: 765–772
11) Nii M, Guerra V, Roman KS, et al: Three-dimensional tricuspid annular function provides insight into the mechanisms of tricuspid valve regurgitation in classic hypoplastic left heart syndrome. J Am Soc Echocardiogr 2006; 19: 391–402
12) Takahashi K, Inage A, Rebeyka IM, et al: Real-time 3-dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation 2009; 120: 1091–1098
13) Knio ZO, Montealegre-Gallegos M, Yeh L, et al: Tricuspid annulus: A spatial and temporal analysis. Ann Card Anaesth 2016; 19: 599–605
14) Ring L, Rana BS, Kydd A, et al: Dynamics of the tricuspid valve annulus in normal and dilated right hearts: A three-dimensional transoesophageal echocardiography study. Eur Heart J Cardiovasc Imaging 2012; 13: 756–762
15) Utsunomiya H, Itabashi Y, Mihara H, et al: Functional tricuspid regurgitation caused by chronic atrial fibrillation: A real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging 2017; 10: e004897
16) Nii M, Roman KS, Macgowan CK, et al: Insight into normal mitral and tricuspid annular dynamics in pediatrics: A real-time three-dimensional echocardiographic study. J Am Soc Echocardiogr 2005; 18: 805–814
17) Ancona F, Stella S, Taramasso M, et al: Multimodality imaging of the tricuspid valve with implication for percutaneous repair approaches. Heart 2017; 103: 1073–1081
18) Maffessanti F, Gripari P, Pontone G, et al: Three-dimensional dynamic assessment of tricuspid and mitral annuli using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 2013; 14: 986–995